1. Background
A prevalence ranging from 5% to 20% has been observed in intensive care unit (ICU) patients developing acute kidney injury (AKI), attributable to diverse factors (1-4). In a study among 1,500,546 children within an integrated health care delivery system in Northern California, the incidence was approximately 1 per 1000 per year, with two-thirds of cases not associated with an ICU admission (5). Plumb et al., in England, using an electronic alert for rising creatinine in hospitalized neonates and children, showed that 8% of AKI occurred during birth admissions and 25% of hospitalized AKI cases required critical care (6). AKI has both short-term and long-term consequences on health. Since 2009, more than 80 cohort studies comprising over 2 million participants from high-income countries have examined the connections between AKI and the risks of cardiovascular diseases, AKI recurrence, hospital readmission, chronic kidney disease (CKD), end-stage kidney disease (ESKD), and mortality. Current research indicates that morbidity and mortality rates are even higher in low- and middle-income countries, especially among children and young adults, and in settings with limited access to dialysis (7-9). Mortality and morbidity associated with renal failure may diverge based on the underlying conditions, such as sepsis or multi-organ failure, as opposed to post-surgical cases, where the highest mortality rates are anticipated (10, 11). Furthermore, studies indicate that pediatric patients treated in non-PICU ICUs exhibit a higher mortality rate, with an odds ratio of 2.56 (12). While acute renal dysfunction may improve in half of the affected individuals, approximately 5% may experience irreversibility, necessitating chronic dialysis or renal transplantation (13). The occurrence of renal dysfunction in ICU-admitted patients can result in prolonged hospital stays, persistent morbidity, and mortality, imposing a substantial financial burden on both patients and healthcare systems in the long term (2, 14). Despite the recognition of acute renal injury (ARI) as commonplace in critical illness and its association with elevated mortality risk, there remains a paucity of epidemiological knowledge regarding AKI in pediatric critically ill patients and the variations in its prevalence across different global regions.
2. Objectives
This study seeks to evaluate the incidence and primary causes of renal dysfunction in pediatric ICU admissions within a tertiary pediatric hospital in Iran.
3. Methods
This investigation constitutes a prospective observational study focused on evaluating the incidence of acute renal failure among pediatric ICU patients in a tertiary pediatric hospital in Iran from September 2019 to August 2020. Ethical considerations were adhered to in accordance with the Declaration of Helsinki, and the study received approval from the ethics committee of Shahid Beheshti University of Medical Sciences, with the ethical code: IR.SBMU.RICH.REC.1401.049.
Inclusion criteria: All patients between 30 days and 18 years of age who were admitted to our PICU during the study period were included in the study.
Exclusion criteria encompassed patients with pre-existing chronic kidney disease stage 5 (glomerular filtration rate < 15 cc/1.73m²/minute) before admission and those discharged within the initial 48 hours from the ICU ward. The pediatric ICU exclusively admitted patients aged between 30 days and 18 years. Patient demographic details, including sex, age, date of birth, ICU admission date, body weight, level of consciousness at admission (based on the Glasgow Coma Scale), primary diagnosis, and history of prior renal diseases, were meticulously documented.
Initial assessments within the first 3 hours involved checking urea, creatinine, and electrolyte levels, while subsequent daily evaluations encompassed the same laboratory parameters. Creatinine measurements were conducted using the Jaffe method, and glomerular filtration rates were determined utilizing the Schwartz formula. Additionally, clinical information, such as daily fluid input and urine output, instances of hypotension, and resuscitation events, were recorded. Outcome measures included length of hospital stay, utilization of mechanical ventilation and vasoactive drugs, prescribed antibiotics during ICU admission, deployment of renal replacement therapy, and mortality, all tracked until the last day of admission in the ICU.
Practically all ICU patients were equipped with a Foley catheter to measure urine volume every 6 hours, with recorded values on the patient's nursing sheet. For individuals lacking a urinary catheter, scaled containers or weighed diapers for infants were utilized. Inclusion criteria involved patients who developed acute renal failure during their ICU stay, as per the AKIN criteria (15). The maximum creatinine level during admission determined the stage of renal failure, categorized as follows:
STAGE I: Increase of 1.5 - 1.9 times from baseline or ≥ 0.3 mg/dL increase within 48 hours, or urine output < 0.5 mL/kg/h for 6 - 12 hours
STAGE II: Increase > 2- to 3-fold from baseline, OR urine output < 0.5 mL/kg/h for 12 hours
STAGE III: Increase > 300% (> 3-fold) from baseline, OR ≥ 4.0 mg/dL with an acute increase of ≥ 0.5 mg/dL or on renal replacement therapy, OR urine output < 0.3 mL/kg/h for 24 hours, or anuria for 12 hours
The primary endpoint of the study was to determine the overall incidence of acute renal failure in ICU patients, while the secondary endpoint aimed to assess the primary causes contributing to this occurrence.
3.1. Statistical Analysis
In the course of statistical analysis, findings were expressed as mean ± standard deviation (SD) for quantitative variables, while categorical variables were presented as frequency (percentage). Continuous variables were compared using either the t-test or Mann-Whitney test, depending on whether data distribution deviated from normal or the assumption of equal variances was compromised across study groups. Categorical variables were compared using the Chi-Square test or Fisher’s exact test as appropriate. To identify the primary baseline determinants of renal failure, a multivariable logistic regression model was employed. Statistical significance was established at P values of ≤ 0.05. The statistical software used for these analyses was SPSS version 23.0 for Windows (IBM, Armonk, New York).
4. Results
This prospective observational investigation enrolled a total of 1,272 consecutive patients admitted to the intensive care units (ICUs) of a tertiary pediatric hospital between September 2019 and August 2020. A total of 127 patients were excluded from the study, including 105 patients due to transfer from the ICU to other departments or death within 48 hours, five patients over the age of 18, and 17 patients with chronic stage 5 kidney failure at admission to the ICU. After applying the exclusion criteria, 1,145 cases were enrolled in the study.
Out of the 1,145 ICU-admitted patients in our referral center, 49 individuals experienced acute renal failure during their hospitalization, resulting in an overall prevalence of 4.3% for renal failure. Among these cases, comprehensive information was available for 46 patients, constituting the final cohort for analysis. The mean age of the participants was 5.10 ± 4.82 years, ranging from 0.2 months to 16 years, with 60.9% being male. The primary reasons for ICU admission included respiratory and cardiac issues (35%), neurological problems such as decreased consciousness and refractory seizures (25%), and post-surgery complications (20%) (see Table 1). The average length of stay for patients with acute kidney injury (AKI) was 7.91 ± 6.44 days, contrasting with a mean total admission duration of 5 ± 1 days.
| Variables | No. (%) |
|---|---|
| Respiratory and cardiac problem | 16 (35) |
| Neurologic problems | 13 (28) |
| Trauma and complications of surgery | 9 (20) |
| Shock | 6 (13) |
| Sepsis and serious infection | 1 (2) |
| Kidney disease | 1 (2) |
| Total | 46 (100) |
Cause of ICU Admission
Among the 46 patients who developed acute kidney injury, the distribution of the maximum stage of renal failure, according to AKIN criteria, was as follows: Stage I in 45.7%, stage II in 19.6%, and stage III in 34.8% during their ICU stay (see Table 2). Approximately half of the patients who developed renal failure did so within the initial 24 hours of ICU admission. Acute tubular necrosis (ATN) emerged as the leading cause, accounting for 60.8% of cases (see Table 3). The causes of ATN included renal ischemia (53%), drug-induced causes (23%), and sepsis (7%).
| Variables | No. (%) |
|---|---|
| Stage I | 21 (45.7) |
| Stage II | 9 (19.6) |
| Stage III | 16 (34.8) |
| The time interval between admission and occurrence of renal failure (day) | |
| 1 | 24 (52.2) |
| 2 | 7 (15.2) |
| 3 | 9 (19.6) |
| 4 | 2 (4.3) |
| 5 | 1 (2.2) |
| 6 and higher | 3 (6.5) |
Causes and Time of Renal Failure
| Variables | No. (%) |
|---|---|
| Prerenal | 10 (21.7) |
| Intrinsic (n = 34) | |
| Acute tubular necrosis (ATN) | 28 (60.8) |
| Nephritis | 5 (10.8) |
| Hemolytic uremic syndrome | 1 (2.1) |
| Postrenal | 2 (4.3) |
| Total | 46 (100) |
Acute Renal Failure Cause (N = 46)
Renal replacement therapy (RRT) was indicated in about a quarter (11) of the patients with renal failure. Among them, six patients received peritoneal dialysis, two patients received hemodialysis, and two patients received continuous renal replacement therapy (CRRT). One patient required dialysis, but the parents did not give their consent, and the patient died. Five of the patients who received peritoneal dialysis and one of the patients who received CRRT died. The reasons for RRT included anuria/oliguria and severe edema in five patients, and severe sepsis (for cytokine removal), electrolyte disturbances, and resistant hypertension in others. Tragically, 18 patients (39.1%) with renal failure succumbed, reflecting a significantly higher mortality rate compared to the general PICU mortality rate of 7.1%.
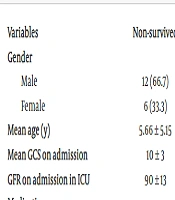
A comparative analysis between the subgroups of survivors and non-survivors with AKI (see Table 4) revealed that non-survivors were more frequently prescribed certain antibiotics, including aminoglycosides and antifungal drugs, compared to their surviving counterparts. Additionally, a history of cardiovascular resuscitation was more prevalent among non-survivors. Notably, the glomerular filtration rate (GFR) at ICU admission did not serve as a predictive factor for in-ICU mortality among the patients.
| Variables | Non-survived | Survived | P-Value |
|---|---|---|---|
| Gender | 0.518 | ||
| Male | 12 (66.7) | 16 (57.1) | |
| Female | 6 (33.3) | 12 (42.9) | |
| Mean age (y) | 5.66 ± 5.15 | 4.74 ± 4.65 | 0.538 |
| Mean GCS on admission | 10 ± 3 | 10 ± 3 | 0.894 |
| GFR on admission in ICU | 90 ± 13 | 92 ± 8 | 0.818 |
| Medications | |||
| Meropenem | 10 (55.6) | 14 (50.0) | 0.713 |
| Vancomycin | 11 (61.1) | 19 (67.9) | 0.639 |
| Cephalosporin | 9 (50.0) | 17 (60.7) | 0.474 |
| Aminoglycoside | 0 (0.0) | 6 (21.4) | 0.035 |
| Anti-viral drugs | 2 (11.1) | 1 (3.6) | 0.552 |
| Antifungal drugs | 5 (27.8) | 1 (3.6) | 0.017 |
| Vasoconstrictors | 10 (55.6) | 15 (53.6) | 0.895 |
| Cardiovascular Resuscitation | 8 (44.4) | 6 (21.4) | 0.048 |
| Mechanical ventilation on admission | 11 (61.1) | 16 (57.1) | 0.790 |
Comparing Parameters Between Survived and Non-survived Patients a
5. Discussion
The occurrence of acute renal failure (ARF) within the pediatric intensive care unit (PICU) presents a complex and critical challenge in pediatric healthcare. The growing attention toward ARF in this specialized setting is driven by its potential for severe morbidity and mortality. The PICU, acting as a hub for conditions such as sepsis, trauma, cardiac anomalies, and nephrotoxic exposures, is a catalyst for the onset of acute kidney injury (AKI). Over the last 15 years, there has been a noteworthy fourfold increase in AKI incidence, coupled with a nearly twofold rise in severe AKI necessitating dialysis, contributing significantly to the upsurge in chronic kidney disease (CKD) incidence (16, 17). Reports over the past decade on AKI incidence in PICU settings have shown a broad range, varying from 1.59% to 82% (18-22). Disparities in AKI incidence can be attributed to differences in study populations, definitions of AKI, study types, observation periods, and markers used to define acute kidney failure. A recent meta-analysis conducted in 2023, encompassing 94 studies, reported an overall AKI incidence of 26% in PICUs. Notably, low-income and low-middle-income countries exhibited higher mortality rates despite a comparable AKI burden (23). In our study, the AKI incidence was 4.3%, aligning with findings from Uchino et al. (1) and Martin et al. (21), who reported AKI prevalences of 5.7% and 4.7%, respectively, following ICU admission. The COVID-19 pandemic has also been associated with an elevated incidence of AKI (24). In this study, 303 patients were examined for acute kidney injury (AKI) using different criteria. The study found that the incidence of AKI was 47.9%, 44.6%, and 50.2% according to the RIFLE, AKIN, and KDIGO criteria, respectively. In-hospital mortality rates were significantly higher in AKI patients across all three criteria, and regression analysis confirmed that AKI was a predictor of in-hospital mortality. Receiver operating characteristic (ROC) analyses showed that each of these criteria had similar abilities to predict in-hospital mortality. The study concluded that the incidence of AKI was higher when using KDIGO criteria, and in-hospital mortality rates were elevated in patients with AKI. Additionally, all three criteria exhibited similar abilities to predict in-hospital mortality (25).
The primary etiology of AKI in our study was acute tubular necrosis (ATN). A 2020 meta-analysis highlighted septic shock as the predominant cause of AKI (26). In the pediatric population, AKI has been independently linked to reduced survival. Our study revealed a mortality rate of approximately 39%, in stark contrast to the general mortality rate of 7% during the same period in the ICU. Notably, the causes of AKI-related mortality differ between adults and children, with the global burden of AKI-related mortality surpassing that of breast cancer, heart failure, or diabetes in the adult population (27). A study by Clermont et al. (28) reported that 8% of ICU-admitted patients suffered from acute renal failure, with 11% requiring hemodialysis and an ICU death rate of 23% in such cases. A study in China by Wen et al. (29) observed acute kidney injury in 31.6% of cases, with a mortality rate of 35.9%, a figure comparable to our study's overall ICU mortality rate. This rate was even higher at about 75% in a recent study on 3,394 children admitted to a PICU in Cameroon (22). According to a 2023 meta-analysis, AKI-associated mortality was noted in 11% of the pediatric population. Mohkam et al. showed that AKI in pediatric patients with COVID-19 involvement led to a 2.5-fold increase in the incidence of mortality (30% vs. 12.6%) (30). Alkandari et al. found that children admitted to the ICU who developed AKI were 4 - 8 times more likely to die than those who did not; severe AKI (KDIGO stage I/III) experienced mortality rates 6 - 10 times higher, even after adjusting for severity of illness and intergroup differences (31). The high mortality rate in our patients may be attributed to our ICU being a tertiary center with many patients having underlying chronic systemic and hereditary diseases.
Klotho levels decreased by vancomycin in a dose-dependent manner, while the expression of reactive oxygen species and antioxidant enzymes increased. This rise in cellular components damages kidney cells and alters renal histology (32). Several factors can predict poor outcomes in AKI. It is evident that the occurrence of various infections and sepsis (leading to septic shock), hemodynamic instability, and cardiovascular involvement can cause kidney impairment and simultaneously increase the risk of death. Oliguric acute renal injury, compared to non-oliguric AKI, and fluid overload versus euvolemic AKI, pose a higher risk for morbidity and mortality in children (33-35).
Chronic kidney disease is an important sequel of AKI. The factors contributing to the transition from acute kidney injury (AKI) to chronic kidney disease (CKD) are not fully understood but are believed to involve ineffective tubular repair, ongoing damage to small blood vessels, and inflammation that ultimately results in the formation of scar tissue (36, 37). Our study identified the use of aminoglycoside and antifungal drugs and a history of cardiovascular resuscitation as significantly more common in non-survivors compared to survivors. Salerno et al. reported that gentamicin + indomethacin were associated with an increased risk of AKI relative to furosemide + tobramycin and vancomycin + piperacillin-tazobactam in infants (38). In a recent systematic review on the incidence and risk factors of vancomycin combined with piperacillin/tazobactam (VPT)-associated AKI in children, it was shown that the incidence of VPT-associated AKI in critically ill children (26.6%) was much higher than in noncritically ill children (10.9%) (39). The age-related protein α-Klotho is encoded by the KL gene. Recent studies suggest that Klotho is beneficial in renal diseases. Some antibiotics, such as vancomycin, decreased the endogenous Klotho levels in vitro and in vivo; notably, it decreased the total Klotho in situ and the soluble Klotho in the plasma. A study by Brivet et al. identified seven variables predictive of death, including advanced age, altered previous health status, hospitalization before ICU admission, delayed occurrence of acute renal failure, sepsis, oliguria, and severity of illness assessed at the time of study inclusion by Simplified Acute Physiology Score, APACHE II, or Organ System Failure (40). Uchino et al. also showed that independent risk factors for hospital mortality included the use of vasopressors, mechanical ventilation, septic shock, cardiogenic shock, and hepatorenal syndrome (1).
Acute kidney injury has also been linked with longer hospital and ICU lengths of stay. Acute kidney injury was associated with a longer hospital stay after adjustment for race, sex, age at admission, clinical diagnosis, and infection (41). In our study, the mean ICU stay was three days longer in AKI patients. Kaddourah et al. showed that AKI patients had a three-day longer hospital stay even after adjusting for the severity of illness (42).
Our study has some limitations. First, due to the relatively small number of patients (46 patients), we did not assess the potential effect of the specific cause of acute kidney injury on patient outcomes. Second, we cannot generalize our findings beyond the pediatric ICU setting or to other pediatric ICUs. Third, we couldn't follow living patients after discharge for signs of persistent kidney damage and chronic kidney disease. Lastly, we did not use a severity scoring system to compare the severity of illness between AKI and non-AKI patients.
This study demonstrated that among all patients hospitalized in the ICU, 4.3% experienced acute renal failure, with a mortality rate of 39%. The occurrence of this disorder mainly transpired during the first days of admission to the ICU, with acute tubular necrosis identified as the primary etiology of acute kidney injury.