1. Background
Home mechanical ventilation (HMV) represents an innovative approach to providing respiratory support in children with various chronic respiratory diseases. Mechanical ventilation is crucial for the stable support of individuals with respiratory failure, particularly those with chronic neuromuscular or neurological disorders (1). Additionally, HMV has been proven to enhance quality of life and reduce hospitalization burdens for these patients (2). By allowing extended ventilation periods, HMV significantly enhances life quality and survival rates for infants and children from one month of age and older (3-5).
The American respiratory care guidelines emphasize several long-term objectives for HMV, such as enhancing life sustainability, improving overall quality of life, reducing complication rates, promoting optimal growth and development, and providing a cost-effective therapeutic option (6). Candidates for HMV include patients aged three months and older (6, 7). The escalating costs of hospital care and advancements in positive pressure ventilation technology for home use have encouraged the transition of patients from hospital settings to home care. Nevertheless, this transition can fail or lead to ICU readmissions if parents are not adequately trained in HMV management or lack sufficient support (8).
The application of HMV varies; for instance, a study in Italy by Ottonello et al. involved 20 children needing long-term mechanical ventilation. Among these, 65% utilized non-invasive ventilation (NIV), while 35% required invasive mechanical ventilation (IMV). Of these, only 10% used their devices during the day, 20% used them for 8 to 12 hours, and the majority, 70%, used them solely at night (9). Given the substantial benefits of HMV and the scarcity of in-depth studies on the pediatric population in Iran, a comprehensive evaluation of this demographic is critical.
2. Objectives
This study aimed to assess the advantages of using home mechanical ventilators at Imam Hossein Children's Hospital.
3. Methods
In this case study, children requiring mechanical ventilation in the hospital for over three months were evaluated by an expert team consisting of a pediatric ICU subspecialist, a pediatric pulmonologist, and a pediatric anesthesiology subspecialist. They were deemed suitable for HMV, following ethical approval by the esteemed Deputy for Research and Technology at Isfahan University of Medical Sciences, under ethical code IR.MUI.MED.REC.1400.577. Inclusion criteria included a willingness to participate, a need for long-term ventilator support, the capability of parents to provide HMV, medical stability, no changes in mechanical ventilation settings and oxygen requirements for two weeks prior to discharge, and parental cooperation. Exclusion criteria encompassed the death of a child during the study, relocation from Isfahan to another region, and parental withdrawal from the study.
Key aspects assessed were the age and gender of the patients, disease prevalence, treatment costs, and a cost comparison between hospital and home care. After patient selection by a pediatric pulmonologist, additional measures included home visits by a nurse and a ventilator company representative when necessary. The oversight and execution of the process involved a team comprising a pediatric pulmonologist, a pediatric resident, a nurse specialized in respiratory care, a physical therapist, a speech therapist, a nutritionist, and a ventilator company technician.
The required equipment included (1) a ventilator, either non-invasive for use with a mask or invasive via a tracheostomy; (2) an oxygen source; (3) a pulse oximeter; (4) an Ambo bag; (5) humidifiers and ventilator accessories for tracheostomy use; (6) a manual suction device, if needed; (7) feeding equipment for non-oral intake; and (8) a first aid kit with resuscitation supplies. Parents and caregivers received comprehensive training on operating the ventilator, suction devices, tracheostomy care (if applicable), nebulizers, and non-oral feeding methods. This training ensured high-quality, effective care. Before discharge, the team assessed and confirmed the caregivers' proficiency in these skills to ensure a safe and gradual transition to home care.
In addition, caregivers received training on strategies to enhance the child's mental and physical health during care. The care team supported both the patient and parents during the transition from hospital to home. The settings of the home ventilator, installed by hospital experts, were fixed and non-adjustable to prevent unauthorized adjustments. The care team educated parents on how to respond to specific alarm situations as necessary. After discharge, nurses provided regular reports, assessments, and tests. Given these factors and the importance of educating parents, efforts were intensified to enhance parents' understanding and proficiency in operating the home ventilator, thereby gradually reducing the frequency of care team visits to the child's home. The frequency of home visits was determined based on the needs expressed by the caregivers and their competence in managing the alarms of the HMV device. For data analysis, descriptive statistics such as mean, standard deviation, maximum, minimum, frequency, and percentage were utilized, employing SPSS software version 23.
4. Results
This study encompassed 20 patients with an average age of 4.092 ± 695.8 years, ranging from 15 months to 16 years. The mean weight of the children was 23.85 ± 14.914 kg, their average height was 115.3 ± 28.2 cm, and the mean BMI was 16.9 ± 6.27 (Table 1). Twelve children (60%) were female, and eight (40%) were male.
| Variables | Min-Max | Mean ± SD | Median (IQR) |
|---|---|---|---|
| Age, y | 1.3 - 16 | 8.695 ± 4.092 | 7 (8.7) |
| Weight, kg | 10 - 64 | 23.85 ± 14.914 | 21.5(16.8) |
| Height, m | 0.7 - 1.52 | 1.153 ± 0.282 | 1.23 (0.43) |
| BMI, kg/m2 | 10 - 32.6 | 16.9 ± 6.27 | 13.74 (8) |
Table 2 indicates that 22.22% of the cases requiring HMV were due to myopathy, 16.67% to sleep apnea, 44.44% to spinal muscular atrophy, and 27.78% to laryngomalacia-tracheomalacia. The distribution of device use based on the method of treatment showed that 30% of the patients utilized tracheostomy, and 70% used mask ventilation. Additionally, 65% of the patients experienced side effects while using the HMV, while 35% reported no side effects. The most commonly reported side effect was dryness or irritation of the eyes. These findings underscore the importance of vigilant monitoring and management of potential side effects associated with HMV use, especially concerning eye health (Table 2).
| Sex | Age | Conditional Disease | Type of HMV | Cost of HMV (Million Rial) | Side Effects | Type of Side Effects |
|---|---|---|---|---|---|---|
| Female | 3.1 | Myopathy | Tracheostomy | 25 | Yes | Abdominal bloating or abdominal pain that causes intolerance to feeding or stopping the use of the device. |
| Female | 15 | Sleep apnea | Musk | 6 | Yes | Abdominal bloating or abdominal pain that causes intolerance to feeding or stopping the use of the device/ Dry or watery eyes. |
| Female | 16 | Spinal muscular atrophy | Musk | 10 | No | - |
| Male | 2.1 | Laryngeomalasy-Tracheomalasy | Musk | 25 | Yes | Intolerance of masks |
| Male | 1.3 | Laryngeomalasy-Tracheomalasy | Musk | 10 | Yes | Dry or watery eyes |
| Male | 5 | Spinal muscular atrophy | Tracheostomy | 60 | No | - |
| Female | 11.5 | Sleep apnea | Musk | 10 | No | - |
| Female | 10 | Spinal muscular atrophy | Musk | 30 | No | - |
| Female | 6 | Spinal muscular atrophy | Musk | 30 | Yes | Intolerance of masks |
| Female | 7 | Laryngeomalasy-Tracheomalasy | Musk | 30 | Yes | Dry or watery eyes |
| Female | 7 | Spinal muscular atrophy | Tracheostomy | 9 | Yes | Abdominal bloating or abdominal pain that causes intolerance to feeding or stopping the use of the device. |
| Male | 10 | Sleep apnea | Musk | 10 | No | - |
| Male | 5 | Myopathy | Musk | 30 | No | - |
| Male | 8 | Laryngeomalasy-Tracheomalasy | Musk | 60 | Yes | Dry or watery eyes |
| Male | 10 | Spinal muscular atrophy | Musk | 6 | Yes | Dry or watery eyes |
| Male | 9 | Spinal muscular atrophy | Tracheostomy | 25 | Yes | Abdominal bloating or abdominal pain that causes intolerance to feeding or stopping the use of the device/Dry or watery eyes |
| Female | 2 | Laryngeomalasy-Tracheomalasy | Musk | 30 | No | - |
| Female | 7 | Spinal muscular atrophy | Tracheostomy | 10 | Yes | Dry or watery eyes |
| Female | 5 | Myopathy | Musk | 15 | No | - |
| Female | 10 | Myopathy | Tracheostomy | 30 | Yes | Abdominal bloating or abdominal pain that causes intolerance to feeding or stopping the use of the device. |
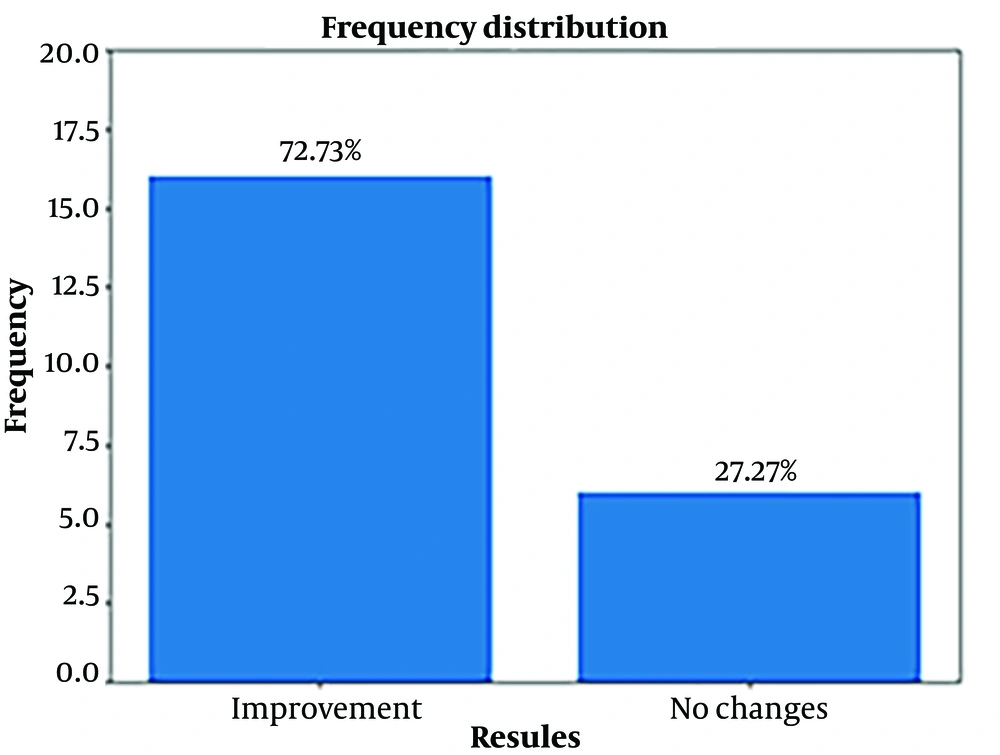
Approximately 73% of patients demonstrated therapeutic effectiveness after utilizing HMV. However, 27% of patients showed no improvement, suggesting that factors such as comorbidities, disease severity, or individual patient characteristics might influence their response to HMV. This underscores the necessity for tailored care and ongoing monitoring (note: The content is a general translation as the exact context of "Figure 1" and the medical condition's specifics are not provided.).
Table 3 reveals that the average cost of re-hospitalization in the ICU decreased significantly by 347.59%, a notable reduction. Similarly, the cost of re-hospitalization in the general ward was reduced by 65.09%.
| Variables | Before Utilizing the HMV | After Utilizing the HMV | Total Change Percent |
|---|---|---|---|
| Ward | 35.95 ± 45.36 | 23.4 ± 32.45 | 65.09 |
| ICU | 280.5 ± 663.92 | 9.75 ± 14.25 | 347.59 |
a Values are expressed as mean ± SD.
5. Discussion
This study was conducted to assess the benefits of using HMV devices at Imam Hussein Children's Hospital. The findings highlight the importance of regular assessments and adjustments to treatment parameters to optimize patient outcomes and provide the highest quality of care. Moreover, continuous advancements in HMV technology and further research are crucial for improving response rates and addressing the needs of patients who do not initially benefit from this treatment. For instance, a study from Massachusetts in 2005 observed that the incidence of long-term ventilation tripled in patients who required respiratory support for over 15 years (10). Additionally, Povitz et al. reported that the annual incidence of mechanical home ventilation authorization increased from 1.8 per 100 000 individuals in 2000 to 5.0 per 100 000 in 2012, marking an annual increase of about 0.3 per 100 000 people per year (11).
Rose et al. reported a 61% efficacy rate for HMV, whereas this study observed an improvement of up to 73% in children using the device. One potential reason for these differing outcomes might be the age range of the participants; Rose et al.'s study included all age groups, which could have affected their results. Additionally, the variability in outcomes might stem from inadequate caregiver training. In this study, caregivers received educational support and regular visits from the care team, enhancing their ability to manage HMV effectively. In contrast, feedback from participants in Rose et al.’s study indicated that only 45% received skills assessments and follow-up training, suggesting that insufficient ongoing education may contribute to inconsistent results (12).
Fauroux et al. (13), Windisch et al. (14), Sterni et al. (7), and Kwak (15) have highlighted that while HMV allows for a more normal daily life compared to institutional or hospital-based ventilation, it also introduces significant psychological, physiological, and social challenges for children and their families. Valko et al. (16) observed a 10.5% improvement in quality of life over six months, underscoring the potential benefits of HMV.
Povitz et al. (11) noted a decrease in the prehospitalization rate from 39.8% to 29.9% after the introduction of HMV, which signifies a substantial reduction in the need for re-hospitalization, falling to less than 90%. This improvement could largely be attributed to the continuous educational support and frequent home visits provided by the care team. Correspondingly, Amin et al. (17) reported that the median length of stay in acute and rehabilitation clinics decreased significantly after tracheostomy insertion, from an initial median of 162.0 days (IQR 98.0 to 275.0 days) to 97.0 days (IQR 69.0 to 210.0 days). Remarkably, in this study, the average duration of hospital stays in intensive care units was reduced from 394 days to just 13 days, illustrating a significant reduction.
The research by Hazenberg et al. (18) suggested that patients using HMV experience fewer side effects and enjoy a better quality of life than others. This aligns with the findings of the current study, where 35% of participants reported no side effects. For the remaining 65%, side effects were typically minor and manageable through education and proper care. However, Mattson et al. (19) noted that "children on mechanical ventilation at home reported a lower quality of life than healthy children and those with other chronic diseases." This disparity may stem from inadequate follow-up and parental support in some cases. A crucial and exemplary aspect of this study was the presence of a supportive care team that began its involvement before discharge, ensuring parents were well-prepared to manage their children's care at home with HMV. Regular follow-up visits were conducted to monitor the child's condition, with ongoing parental support until they felt confident in their caregiving abilities.
Regarding medical costs, this study found that hospital expenses for patients totaled 349.05 million rials, which significantly decreased to 34.7 million rials with home care. Similarly, Ottonello et al. (9) reported, "We compared the cost of home care with the actual hospital costs (1324 €/day in the intensive care unit of the G. Gaslini Children's Hospital) and found a significant economic advantage of home care (7593 €/week)."
5.1. Study Limitations
A key limitation of this study was the absence of a control group. It is suggested that future research replicates this study as a clinical trial to better control confounding variables and strengthen the generalizability of the findings.
5.2. Conclusions
This study has provided valuable insights into the use of HMV for patients requiring long-term ventilation. The findings indicate the effectiveness of HMV in improving health outcomes for the majority of patients, significantly reducing medical treatment costs, and shortening hospital stays. These results underscore the importance of HMV and suggest the need for more detailed research in this area. Considering the growing number of children requiring HMV, these findings can guide the development of essential support programs for these patients and their families.