1. Background
Intravenous immunoglobulin (IVIG) is an immunoglobulin G preparation obtained by pooling from thousands of healthy donors (1). It is believed to show its therapeutic effect by inactivating bacterial exotoxins and endotoxins, leukocyte stimulation, and increasing serum bactericidal activity, and immunoglobulins have regulatory and suppressive effects on cytokine release (1, 2).
Intravenous immunoglobulin has various clinical applications and is commonly used in neonatal intensive care units to treat alloimmune hemolytic disease of the newborn (AHDN) (3, 4). However, the usefulness of IVIG for sepsis treatment and prophylaxis has been debated (4). A systematic review published in 2015 concluded that IVIG is ineffective in sepsis prevention or treatment (5).
Regarding the use of IVIG in AHDN treatment, there have been some concerns about bias in the published studies supporting its use (3). However, overall, IVIG is still considered a standard treatment for AHDN in many studies, which suggests its effectiveness in reducing the need for blood transfusions and improving outcomes in affected infants (6-8). It is reported that IVIG decreases the rate of hemolysis and the need for exchange transfusion in AHDN treatment, and it demonstrates its effectiveness by binding to Fc receptors and blocking circulating maternal antibodies (9).
While IVIG is generally considered safe, adverse events, such as pyrogenic reactions (10), volume loading (11, 12), hypoglycemia (11), and hypotension (10), might occur. In older children with IgA deficiency or hypogammaglobulinemia, hypersensitivity and anaphylactic reactions are possible, secondary to anti-IgA antibodies (9). With advanced purification processes, the probability of transmitting infectious diseases with current IVIG products is very low. However, rare complications, such as hemolysis, acute renal failure, necrotizing enterocolitis (NEC), and severe gastrointestinal system (GIS) complications requiring surgical interventions, have been reported (13-16), which might cause death (13).
2. Objectives
Intra-abdominal perforations are life-threatening complications; however, there are no data available regarding associated risk factors. This study aims to investigate the relationship between the immunoglobulin A (IgA) levels of IVIG preparations and the occurrence of intra-abdominal perforation or gastrointestinal bleeding following IVIG infusion in newborns.
3. Methods
This study included patients who received IVIG therapy in 4 years between 2017 and 2020 in the neonatal intensive care units of Marmara University and Gungoren hospital, Istanbul. Turkey.
Patients with gastrointestinal bleeding or intra-abdominal perforations without any underlying diseases except AHDN were included. Patients born small for gestational age, with intrauterine growth retardation, preterm-born patients, congenital anomalies interfering with GIS passage, and congenital heart defects were excluded. This retrospective study received permission from the Marmara University Ethics Committee (file number: 09.2022.276).
We documented birth week, weight, gender, and delivery type. The IgA levels of IVIG preparations used in the current patients were obtained from the prospectus information. Intravenous immunoglobulin infusion therapy was administered 1 g/kg over 4 hours.
This study included infants born ≥ 35 gestational weeks and excluded those before 35 weeks to distinguish the cases that underwent surgery due to simple NEC, which is more likely to occur.
This study used descriptive statistics, such as mean, median, standard deviation, and interquartile, to summarize continuous variables in the present dataset. This study used the Mann-Whitney U test as a nonparametric statistical test to compare two independent groups when the dependent variable is not normally distributed and the Student's t-test as a parametric test for normally distributed data. Fisher's exact test was used to analyze contingency tables with categorical variables when the expected count is less than five. This study used receiver operating characteristic (ROC) analysis to assess the reach of the best area under the curve (AUC). Additionally, to assess the evidence, the study used Bayesian calculations with H1 (association hypothesis) and H0 (non-association/independence hypothesis), ran the calculation using Bayesian Kendall test by setting the stretched beta prior width to 1, and presented Bayes factors as BF10. The threshold for statistical significance was set at p < 0.05. The Jamovi 2.3.18 statistical package program was used for statistical calculations with jsq and psychoPDA extensions.
4. Results
A total of 72 patients received IVIG therapy, and 15.5% (n = 11) developed major GIS complications. However, 36 subjects were born later than 35 gestational weeks, and the severe complication rate was 22.2% (n = 8) in this group. The descriptive data are presented in Table 1. None of our patients was born small for gestational age or had intrauterine growth retardation.
| Variables | Non-complicated (n = 28) | Complicated (n = 8) | P-Value |
|---|---|---|---|
| Birth week | 38.3 (37.6 - 39.4) | 39 (38 - 39.1) | 0.159b |
| Birth weight | 2878 ± 554 | 3256 ± 519 | 0.317c |
| Delivery | |||
| NSD | 35.7 (n = 10) | 25% (n = 2) | 0.691d |
| C/S | 64.3% (n = 18) | 75% (n = 6) | |
| Gender (female) | 46.4% (n = 13) | 62.5% (n = 5) | 0.691d |
| IgA level (mg/dL) | 4 (2.5 - 20) | 20 (14 - 20) | 0.024b |
| Postnatal day | 2 (1 - 4) | 3 (1 - 8) | 0.649b |
Abbreviations: NSD, normal spontaneous delivery; C/S, cesarean section.
a Data are presented as mean ± SD, median (interquartile range), or No. (%).
b Mann-Whitney U test.
c Student’s t-test.
d Fisher’s exact test.
We could not reach the records of three patients about the IVIG pharmaceutical brand. Severe GIS complications (perforation/massive GIS bleeding) occurred in 22.2% (n = 8) of the patients. Six patients had gastrointestinal perforation based on radiological imaging, and the location of the perforation was apparent from surgical and per-operative pathological findings in five patients. One patient died before surgery due to the severity of the clinical course. Two patients had lower gastrointestinal bleeding and NEC without any prior gastrointestinal symptoms or predisposing factors. It is also important to note that none of them had congenital heart disease, intrauterine growth retardation, severe sepsis, congenital anomalies interfering with GIS passage, or perinatal asphyxia history, which might have confounded the results.
There were no statistically significant differences in terms of the birth week (P = 0.159, Student's t-test), birth weight (P = 0.317, Student’s t-test), delivery type (P = 0.691, Fisher’s exact test), postnatal day (P = 0.569, Mann-Whitney u test), and gender (P = 0.691, Fisher’s exact test).
A total of 31 term newborns in our clinics received IVIG therapy. Three of these patients (9.7%) developed complications related to gastrointestinal perforation or bleeding. One patient who was given IVIG in our clinic due to AHDN died due to gastrointestinal perforation, and two patients had lower gastrointestinal bleeding that eventually recovered. Among other complicated patients, five were referred to our clinic, and three could not survive after gastrointestinal perforation. Two of them were given IVIG due to sepsis, and three of them due to AHDN. A total of six patients had intestinal perforation, and four of them died after the irreversible phase of intra-abdominal sepsis. The clinical features and survival status of the complicated cases are presented in Table 2.
| Clinical Features | Survival | Indication | Diagnosis | PN Day | BW | GW |
|---|---|---|---|---|---|---|
| Diffuse necrosis in terminal ileum and colon | Deceased | AHDN | Intraop. | 1 | 2000 | 35 |
| Lower GIS bleeding, Bells stage 1B | Survived | AHDN | Clin/Rad | 8 | 3070 | 38 |
| Sub-diaphragmatic free air | Deceased | AHDN | Clin/Rad | 1 | 3120 | 40 |
| Sub-diaphragmatic free air | Deceased | Sepsis | Clin/Rad | 3 | 4110 | 36+3/7 |
| Gastric perforation | Deceased | Sepsis | Intraop. | 20 | 3030 | 38 |
| Lower GIS bleeding, Bells stage 2B | Survived | AHDN | Clin/Rad | 1 | 3290 | 39+1/7 |
| Necrosis on the colon and SIP at the terminal ileum | Survived | AHDN | Intraop. | 8 | 2000 | 36+4/7 |
| SIP on the transverse colon | Survived | AHDN | Intraop. | 3 | 3390 | 36+4/7 |
Abbreviations: GW, gestational week (week); BW, birth weight (gr); PN day (days), postnatal day of treatment; Intraop., Intraoperative diagnosis; Clin/Rad, clinical and radiologic diagnosis; AHDN, alloimmune hemolytic disease of the newborn; GIS, gastrointestinal system; SIP, spontaneous intestinal perforation.
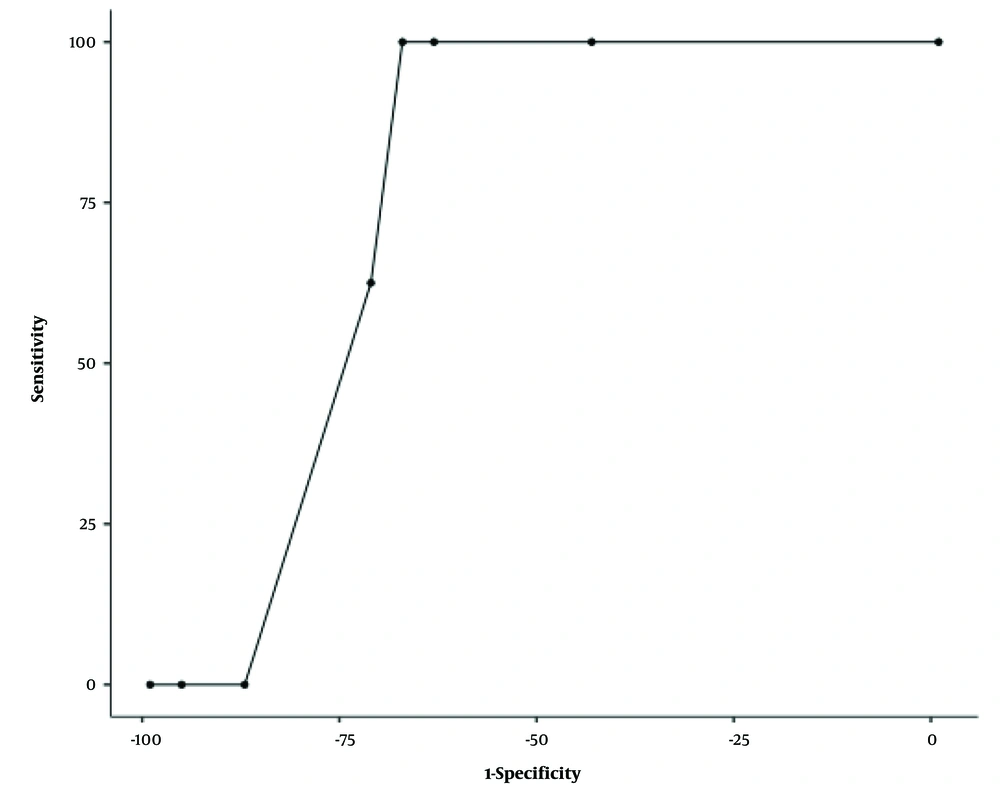
As the present study compared the IgA levels of the IVIG preparations, the GIS perforation/bleeding group had statistically significantly higher IgA levels than the non-complicated group (4 mg/dL [2.5 - 20] vs. 20 [14 - 20]; P = 0.024, Mann-Whitney U test). This study built an ROC analysis regarding IgA levels and major GIS problems. The cut-points 5 mg/dL and 14 mg/dL resulted in statistically significant thresholds with an acceptable 0.762 AUC and 100% sensitivity and negative predictive value (Table 3, Figure 1).
| Cut-point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden's index | AUC | Metric Score |
|---|---|---|---|---|---|---|---|
| 5 mg/dL | 100% | 64% | 47.06% | 100% | 0.64 | 0.762 | 1.64 |
| 14 mg/dL | 100% | 68% | 50% | 100% | 0.68 | 0.762 | 1.68 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
This study built the H1 hypothesis for factors that might affect major GIS complications, and the H0 hypothesis might not affect (independence from the GIS complications hypothesis) using Bayesian calculations. Postnatal days and birth weight had moderate evidence; gestational week and gender had anecdotal evidence for independence from GIS complications where IgA levels had strong evidence for the H1 hypothesis (relation with GIS complications), and correlation resulted in P < 0.05 (r = 0.362, Kendall’s Tau b). This study set a threshold of 14 mg/dL for IgA (as a result of ROC analysis) and built two groups. Furthermore, the correlation coefficient resulted as r = 0.583 with a stronger correlation (P < 0.001), and the Bayesian statistics resulted in excessive evidence (BF10 > 1000) (Table 4) in this new model.
Abbreviations: GIS, gastrointestinal system; IgA, immunoglobulin A; AI, anecdotal evidence for independence hypothesis; MI, moderate evidence for independence hypothesis; S, strong evidence for H1 hypothesis; S, strong evidence for H1 hypothesis; BF, Bayes factor.
a P < 0.05.
b P < 0.001.
5. Discussion
Intravenous immunoglobulin is in clinical use for treating hemolysis if the infant is at the exchange-transfusion border (6-8); however, there are also adverse effects (10-12). We administered IVIG to three patients with this indication. Among the referred patients, three were administered IVIG due to AHDN and two due to sepsis.
While IVIG might be effective in treating AHDN (9), factors such as age, overall health, and severity of the condition should be considered when making treatment decisions, as it might cause side effects (9), even the risk of NEC is a vital concern and healthcare providers must be aware of this potential complication when considering IVIG treatment (17, 18). Therefore, patients need to be monitored closely. Intravenous immunoglobulin could change blood viscosity and cause alterations in cytokine release. These two mechanisms might contribute to NEC pathology (17). However, the importance of careful monitoring and slow infusion rates can help minimize the risk of hyperviscosity and other potential side effects associated with IVIG therapy (17).
Ischemia and hypoxia of the intestinal tissues are the primary factors leading to the development of NEC. Coagulation necrosis is observed in the histopathological study of intestinal tissue obtained from infants affected by NEC (19). In vitro studies have shown that blood viscosity increases with increasing immunoglobulin levels (20). According to another hypothesis, IVIG increases interleukin and tumor necrosis factor-alpha release, and interleukin-1 causes a contraction in intestinal vessels by altering the expression of nitric oxide synthase, change in blood flow due to increased viscosity, leading to mesenteric ischemia, bowel distention, intestinal necrosis, bacterial overgrowth, and translocation, and eventually, NEC occurs (18). Therefore, IVIG treatment should be given slowly to minimize the effects of hyperviscosity, and newborns should be continuously monitored for all possible side effects, especially thrombotic events (13).
On the other hand, despite the increased risk of NEC associated with IVIG infusion, a meta-analysis has suggested that it does not affect mortality in preterm neonates (18). Due to another hypothesis, IVIG might increase the platelet count (18). Navarro et al. (15) observed venous thrombosis in NEC cases and detected micro mesenteric vein thrombosis in intestinal resection material on pathological examination after IVIG administration. Additionally, IVIG might increase the prothrombotic effect by increasing physiological hypercoagulability in the first days of life (13). Studies in preterm infants suggested that the flow velocity change measured by the mesenteric artery Doppler is related to NEC (19). However, superior mesenteric and celiac artery blood flow did not alter significantly between immediately and after 12-18 hours of IVIG infusions (1 g/kg) while treating AHDN and neonatal alloimmune thrombocytopenia (21). However, it is crucial to note that while thromboembolic events have been reported in adults after IVIG infusions (22-24), the risk in neonates might be different due to differences in physiology and dosing.
In this study, there was a clinical observation that major gastrointestinal problems might develop more frequently due to the high level of IgA in the drug content in patients given IVIG treatment; therefore, we searched the literature regarding severe complications regarding IVIG infusion and identified similar cases reported in the literature which made us think there might be side effects related to IgA levels which could not be entirely distilled resulting remaining of little IgA amounts. Additionally, the IgA contents in IVIG preparations might vary (2), which was the initial step for us to speculate that IgA levels in IVIG preparations might increase the likelihood of major gastrointestinal problems. Immunoglobulin A levels in IVIG preparations are critical in preventing immunization and treatment-related reactions (25). Navarro et al. (15) reported that 0.5 g/kg single dose, 1 g/kg single dose, and 1 gr/kg 2 doses of IVIG preparations containing less than 130 mcg/mL IgA levels (26), which they gave with phototherapy for AHDN, caused NEC in two patients and intra-abdominal perforation in one patient. Krishnan and Pathare (16) reported that NEC and intra-abdominal perforation in a term newborn resulted in death after IVIG infusion given for treating AHDN, which included 400 mcg/mL of IgA content.
Additionally, there are two studies regarding older children with various diseases that declared perforation after IVIG treatment in the literature. One study reported that a 2-year-old patient with duodenal perforation during a multisystem inflammatory syndrome course developed a second duodenal perforation after IVIG therapy (27). Another 2.5-year-old patient with Kawasaki disease suffered perforation in the descending duodenum after IVIG treatment (28). It is a fact that both systemic diseases might affect intestinal circulation; however, this rare complication, GIS perforation, occurred after IVIG treatment. Figueras-Aloy et al. (13) suggested that the use of high-dose IVIG should be carefully considered and monitored in newborns with AHDN, especially in those born at or above 34 gestational weeks who are receiving phototherapy as NEC developed in 6% of them, and 40% of NEC cases required urgent surgery and caused the death of two patients (13). In the current study group, 31 newborns in our clinic were treated with IVIG, three patients (9.7%) developed severe GIS complications, and one of them had gastrointestinal perforation and died as a result of these complications. Five patients were referred to the clinic specifically for gastrointestinal perforation, and three of these patients died due to the perforation.
In the neonatal period, intestinal perforation might develop spontaneously or secondary to NEC or mechanical obstruction. Spontaneous intestinal perforation (SIP) means developing perforation in a region of the gastrointestinal tract for no apparent reason. This region is typically the terminal ileum. It is observed more frequently in low and very-low-birth-weight preterm and rarely in term neonates. Etiology and pathogenesis are not yet known. Fetal or perinatal asphyxia in history or follow-up is of importance (29). Stress, hypoxia, and circulatory failure can cause regional hypoperfusion. Temporary ischemia and reperfusion can cause SIP. Spontaneous intestinal perforation is the second most common intestinal perforation after NEC. In exceptional cases, perforation can occur in more than one area. Spontaneous intestinal perforation differs from NEC in the absence of inflammation (30). In the present study group, two patients at corrected gestational ages of 37 weeks and 37 + 5/7 weeks had spontaneous localized ileal perforation, a general finding observed in SIP cases. In the operation, resection and anastomosis of the perforated area were performed. Although SIP is a rare condition, clinicians should have a high level of suspicion for SIP in neonates who develop gastrointestinal symptoms because prompt recognition and management are crucial.
The present study could not reach other IVIG preparations or IgA levels, resulting in NEC and intra-abdominal perforation in the databases. In the current patient group, the main finding was that the IgA levels of the IVIG preparations were statistically significantly higher in the GIS perforation/bleeding group, and the perforation rate was higher in > 14 mg/dL than in the ≤ 14 mg/dL group.
5.1. Conclusions
The complication rate is a bit high in the current patient group; however, it is crucial to be aware of the potential gastrointestinal complications of IVIG therapy, including NEC, SIP, and lower gastrointestinal bleeding, and to monitor newborns closely for any signs of adverse effects. The use of IVIG therapy should be carefully considered, and the choice of IVIG preparation with low IgA content might be beneficial in reducing the risk of adverse gastrointestinal effects. Further extensive and prospective studies are necessary to identify risk factors and optimize the use of IVIG therapy in neonates with AHDN and other conditions. It is also essential to inform patients and their families of the potential risks and benefits of this treatment so that they can make informed decisions about their care.