1. Context
Spinal muscular atrophy (SMA) is a genetic neuromuscular disorder that results in the loss of alpha motor neurons in the spinal cord and brainstem, severely impacting patient mobility, respiratory functions, life expectancy, and quality of life (1). Clinically, this disease is divided into four subcategories: SMA type 1, SMA type 2, SMA type 3, and SMA type 4, identified by the time of symptom onset and degree of mobility. There are no known permanent treatments for SMA; available treatments focus on delaying symptom onset and severity. However, since the breakthrough discovery of SMA’s genetic causes, the quality of care, treatment options, and efficacy for these patients have been revolutionized (1, 2). Due to SMA’s chronic and progressive nature, care for these patients must be patient-oriented, multi-sectoral, and long-term. Beyond prescriptions, their care package typically includes physical therapies, dietary counseling, respiratory assistance, and palliative care. This requires a diverse set of technologies and professionals, including pulmonologists, orthopedists, physical therapists, and dietitians, all usually led by a neurologist.
Spinal muscular atrophy is a costly disease, but the cost of care for these individuals is not merely an accumulation of drug vials, hospitalization, and specialized care costs. Many of their needs are met informally by family members, unpaid nurses, or caretakers. Additionally, treatment costs vary by disease type. Patients with SMA types 1 and 2 mostly rely on hospitalization, specialized care, and medical consultations, leading to higher initial costs, whereas treatment costs for patients with SMA types 3 and 4 accumulate over their lifetime. According to a study from Germany, 67% of type 1, 17% of type 2, and only 7% of type 3 patients required hospitalization. Additionally, 100% of type 1, 96% of type 2, and 79% of type 3 patients needed rehabilitation services (2). Studies from Spain estimated an annual cost of €34,000 per patient, comprising 32.3% direct costs and 67.7% indirect costs (3). This is not something patients or their families can easily afford, so they often heavily rely on financial aid from insurance institutions and governments, which can sometimes be hesitant to provide support.
One way to reassure these supporting bodies of the efficacy and cost-effectiveness of coverage for these orphan drugs and care packages is through economic evaluations. Economic evaluation enables payers, such as insurance companies, to assess the cost-effectiveness of a new treatment while considering its health benefits/risks compared to an established standard of care. Even though economic evaluations for SMA treatments face unique challenges—such as a lack of consensus on an evaluation model or index, insufficient local data on patients and their costs, and difficulties in assessing added value—due to its flexible nature, it is still the best approach (4).
According to reported estimations, there are currently 700 to 800 patients registered with SMA in Iran (5, 6). This paper aims to gather the current understanding of the safety, efficacy, and economic aspects of common SMA treatments to bridge the knowledge gap and assist Iranian policymakers in adopting these novel treatments for this vulnerable population.
2. Methods
This is a rapid health technology assessment (HTA) report to gather and analyze interventions related to SMA. In this type of rapid assessment, the main themes are the identification of primary indications, safety and effectiveness conditions, and an overview of economic aspects. To achieve this objective, the study was conducted in four steps: (1) database review; (2) screening; (3) data extraction; and (4) thematic analysis.
The database review concluded on January 26, 2023. The search strategy combined the following queries:
#1) SMA OR “Spinal Muscular Atrophy”
#2) Efficacy
#3) Safety
#4) #1 AND #2
#5) #1 AND #3
#6) #4 OR #5
The inclusion criteria consisted of studies focused on assessing the safety, efficacy, and economic aspects of interventions in SMA patients compared to those who did not receive such interventions. All types of studies (clinical trials, HTA, reviews, etc.) were included. Exclusion criteria included studies in languages other than English and animal studies. Initially, 1600 studies were identified, and after applying the inclusion and exclusion criteria, 10 entered the final phase. The PICOD was designed as follows: Population (P); SMA patients (types 1 to 4), intervention (I); all treatment interventions (medicines), control (C); without receiving any treatment, outcome (O); side effects, clinical outcomes, and health benefits, design (D); health technology assessment reports, systematic reviews, and clinical trials.
Finally, based on the main rapid assessment themes, data extraction was performed from the designed and retrieved data and analyzed through a qualitative format.
3. Results
From the 1600 initially retrieved studies, 10 were included in our research after screening (Table 1). Three main treatments were identified: Nusinersen, onasemnogene abeparvovec, and risdiplam. Additionally, a combination of nusinersen and onasemnogene abeparvovec was used in many cases.
| No. | Title | Year | Study Type |
|---|---|---|---|
| 1 | Expert recommendations and clinical considerations in the use of onasemnogene abeparvovec gene therapy for spinal muscular atrophy. | 2020 | Experts’ opinions |
| 2 | How should we measure quality of life impact in rare disease? Recent learnings in spinal muscular atrophy. | 2019 | Experts’ opinions |
| 3 | Systematic literature review to assess the cost and resource use associated with spinal muscular atrophy management | 2021 | Systematic review |
| 4 | Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. | 2020 | Randomized controlled trial |
| 5 | Assessing the value of nusinersen for spinal muscular atrophy: A comparative analysis of reimbursement submission and appraisal in European countries. | 2021 | Review |
| 6 | Systematic literature review to assess economic evaluations in spinal muscular atrophy [SMA]. | 2022 | Systematic review |
| 7 | Nusinersen for adolescents and adults with spinal muscular atrophy: A review of clinical effectiveness | 2020 | Review |
| 8 | Nusinersen treatment of spinal muscular atrophy: Current knowledge and existing gaps | 2018 | Review |
| 9 | Evidence-based review of newborn screening for spinal muscular atrophy [SMA]: Final report (v5.2) | 2018 | Systematic review |
| 10 | Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy [SMA] treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: A systematic review of real-world study data | 2022 | Systematic review |
3.1. Safety
3.1.1. Nusinersen
Side effects related to this drug were reported in several studies. In Yeo et al.'s study (as cited by Janoudi and McCormack), a total of 12 complications were observed, three of which were acute (two related to bedsores and one related to falling out of bed). In Hagenacker et al.'s study (as cited by Janoudi and McCormack), 47% of patients experienced side effects, none of which were acute, and in another study, 14% of patients experienced side effects. Often, post-lumbar puncture syndrome occurred without closure of the puncture site, leading to temporary leakage of CSF and headaches (in 15%, 7%, and 2% of patients, respectively). One study reported serious adverse events in 64% of patients, but it was unclear whether these were drug-related. Side effects were mainly related to lumbar puncture, including headaches, post-lumbar puncture syndrome, nausea, and vomiting, which occurred in 20 - 40% of patients (1, 7, 8). Other side effects mentioned by the studies were mild and often included fever, cough, pneumonia, and other early respiratory tract infections (2). Nusinersen showed a favorable safety profile with no evidence that the treatment caused drug-related side effects (4).
3.1.2. Onasemnogene Onasemnogene Abeparvovec (Zolgensma®)
Only one study reported adverse events. Of the 275 complications, 53 (19%) were serious; however, only two of these were related to the treatment intervention itself (9). The use of this drug in high doses for patients with liver problems, thrombocytopenia, weak children with difficulty in swallowing, and other underlying diseases or patients with anti-AAV9 antibodies may pose serious risks (3, 4).
3.1.3. Combined Treatments Nusinersen - Onasemnogene Abeparvovec
Eight serious adverse events were reported in one study, none of which were related to the treatment intervention. However, another study reported an increase in liver enzymes and liver damage due to the use of onasemnogene abeparvovec, which was attributed to the drug. No complications were reported for the use of nusinersen (9). The only possible complication for nusinersen treatment is thrombocytopenia (3). In general, compared to other drugs, nusinersen was found to be the safest treatment method with the fewest side effects for SMA patients.
3.2. Efficacy
3.2.1. Nusinersen
Nusinersen was the first SMA drug to be approved by the American, European, and Canadian FDAs for SMA types 1 and 2 (4). This medication is administered in multiple doses through intraspinal injection (four initial injections, followed by three annual maintenance injections) (4). Nusinersen was found to be effective in reducing reliance on ventilation and dietary support in SMA type 1 patients and increasing mobility achievements in SMA type 2 patients. There is insufficient clinical evidence to prove nusinersen’s effectiveness for SMA types 3 and 4. Due to its proven positive outcomes, nusinersen has been covered by insurance in many countries (3, 4).
3.2.2. Onasemnogene Abeparvovec
Onasemnogene abeparvovec was the second drug approved by the FDA and is administered as a one-time intravenous injection. Onasemnogene abeparvovec has been proven to positively impact reducing ventilatory and dietary support needs and improving mobility in SMA type 1 patients (3, 4). Like its predecessor, onasemnogene abeparvovec is also covered by insurance plans in many countries.
3.2.3. Nusinersen - Onasemnogene Abeparvovec
This combined treatment is typically used for patients who continuously need ventilatory and dietary support and show little mobility improvement while receiving either drug alone. Reported side effects and dangers of this treatment were similar to those of onasemnogene abeparvovec. This treatment helps patients sit independently and move their head and neck (2).
3.2.4. Risdiplam
Risdiplam was the latest drug to receive FDA approval for SMA types 1, 2, and 3. It is an oral liquid consumed daily by patients aged 2 months and older (3, 4). No studies of this drug met the inclusion criteria for this review (10). Table 2 summarizes our findings.
| Treatments | Side Effects | Efficacy |
|---|---|---|
| Nusinersen | Post lumbar puncture syndrome, bed soreness headaches,other symptoms such as: Fever, coughing,other respiratory infections as ,were possible side effects as well. | Reduced reliance on ventilation and dietary support in SMA type 1 patients,increased mobility achievements in SMA type 2, no sufficient clinical evidence for SMA type 3 and 4 patients |
| Onasemnogene abeparvovec | Dangerous for patients with: Liver and swallowing problems, thrombocytopenia, Aav9 antibody, other underlying diseases | Reduced ventilatory and dietary support needs, Improving mobility in SMA type 1 |
| Nusinersen - onasemnogene abeparvovec | Similar to onasemnogene abeparvovec. | Helped SMA type 1 patients to sit independently and move their head and neck, SMA types 2 to 4 were stable and had little improvements |
| Risdiplam | N/A | N/A |
Even though these drugs have shown limited improvements, nusinersen was found to have the greatest overall impact on patients. However, treating SMA depends on many variables, including the timing of treatment (before or after symptom onset) and the specific type of the disease.
3.3. Economic Evaluation
There are few economic evaluations of care costs for these patients, but estimates illustrate that the cost of care for SMA types 1 and 2 is higher than for types 3 and 4, and costs are also higher in the USA than in Europe (3). One recent study from Germany reported that the majority of service costs for these patients are for medical consultations, hospitalization, and artificial and supplementary nutrition (2). The average annual costs for these patients start at €6,696 for types 2 and 3, and €76,935 for type 1 patients. Additionally, reported informal costs for these patients were much higher, starting at €27,636 for type 3 and €60,122 for type 1 (3).
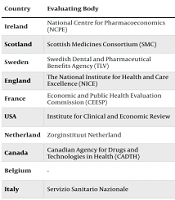
It is estimated that SMA patients in Iran have an annual cost of $52,631 per patient (calculated with currency exchange price of 28500 Rials). According to reports, risdiplam (marketed as Evrysdi) and nusinersen (marketed as Spinraza) are the two treatments currently available for SMA patients in Iran. These drugs are currently directly covered by the Iran Health Insurance Organization, but this is only a six-month plan, and further coverage decisions will depend on additional cost-effectiveness and economic evaluations (3, 5, 6, 10) (Table 3).
| Country | Evaluating Body | Results (in € Per QALY a ) | Cost Effectiveness | Coverage Offered |
|---|---|---|---|---|
| Ireland | National Centre for Pharmacoeconomics (NCPE) | €512844 for SMA type 1 to €2156624 for SMA types 2 and 3 per each QALY | Not cost-effective | MEA |
| Scotland | Scottish Medicines Consortium (SMC) | €508537 for SMA type 1 to €1926381 for SMA types 2 and 3 per each QALY | Not cost-effective | Through NHS |
| Sweden | Swedish Dental and Pharmaceutical Benefits Agency (TLV) | €17142 for SMA type 1 to €322858 for SMA type 2 and €1564889 for SMA type 3 3 per each QALY | Not cost-effective | MEA |
| England | The National Institute for Health and Care Excellence (NICE) | €492350 for SMA type 1 to €1513499 for SMA types 2 and 3 per each QALY | Not cost-effective | Through NHS (CED) b |
| France | Economic and Public Health Evaluation Commission (CEESP) | €950380 for SMA type 1 to €2719821 for SMA types 2 and 3 per each QALY | Not cost-effective | MEA |
| USA | Institute for Clinical and Economic Review | €608176 for patients prior to symptom onset €1037257 for SMA type 1 to €7607792 for SMA types 2 and 3 per each QALY | Not cost-effective | MEA (depending on insurer) |
| Netherland | Zorginstituut Netherland | €632802 for SMA type 1 to €1792939 for SMA types 2 and 3 per each QALY | Not cost-effective | Pay for service (11) |
| Canada | Canadian Agency for Drugs and Technologies in Health (CADTH) | €464891 for SMA type 1 to €2153470 for SMA type 2 and €1994746 for SMA type 3 per each QALY | Not cost-effective | Covered under specific conditions |
| Belgium | - | No available economic evaluation | Not cost-effective | Pay for service (11) |
| Italy | Servizio Sanitario Nazionale | €3385.49 for SMA type 1 to €1828.23 for SMA type 2 and €767.20 for SMA type 3 per each QALY | Not cost-effective | Covered by government |
| Germany | - | €621354 for SMA types 1 and 2 for the first year and €310878 to €310943 for next years. | Not cost-effective | |
| Iran | Iran Health Insurance Organization | Estimated to be 52631 $ annually (not adjusted per QALY) | Not cost-effective | Covered by government |
a Quality adjusted life years.
b Coverage with evidence development.
4. Discussion
Spinal muscular atrophy is a rare disease with no current cure. The available treatments focus on alleviating pain, increasing life expectancy, enhancing quality of life and independence, and delaying symptom onset. According to reports, nusinersen showed the best results with minimal side effects for SMA patients, followed by onasemnogene abeparvovec as the second most effective treatment, and their combination as the third. No sufficient data was found regarding the efficacy of risdiplam (9, 12).
Despite limited data, all three treatments were found to be not cost-effective when compared to the standard of care. However, they were all reimbursed by various countries. Some countries covered the treatments through governmental means (e.g., NHS), while many chose to reimburse them using managed entry agreements (MEAs) (2-4, 10). Managed entry agreements are a varied group of agreements used as flexible tools to negotiate price and reimbursement deals for novel drugs and health technologies between pharmaceutical/health technology companies and service providers, payers, or market regulatory bodies. The primary goal of MEAs is to create affordable and appropriate access to potentially life-saving new interventions (13).
Spinal muscular atrophy patients face numerous formal and informal costs that can accumulate and become significant barriers to access, increasing their unmet needs. On one hand, these patients require a diverse range of specialized services, including dietary and respiratory assistance, physical therapy, orthopedics, and medical consultations. On the other hand, they encounter informal costs related to commuting to and from hospitals, acquiring medical equipment, receiving education, and adjusting their lifestyles and places of residence. These expenses are in addition to the high cost of each drug vial used for their treatment, resulting in a substantial overall cost of care. Many of these costs are not reimbursed or even formally recognized by authorities, making financial support, insurance coverage, and reimbursements crucial for their treatment (1, 3, 14).
Decisions regarding the reimbursement of a drug are usually based on its added value, patient need, and economic evaluation. However, this formula is not suitable for orphan drugs, as many rare diseases, especially genetic ones, are unlikely to be cured, and treatments focus on alleviating symptoms and improving patients’ quality of life and life expectancy. Furthermore, orphan drugs are designed for a small portion of the population, making them pricier than common drugs and lacking economic justification. They are not cost-effective for pharmaceutical industries or service providers. This dissuades many players in the health sector from providing the required care and shifts their focus to other, easily treatable, and cost-efficient illnesses.
Most reimbursement decisions for orphan drugs are based on factors such as the lack of alternative treatments, the significant impact of the novel drug compared to its counterparts, patients’ level of unmet needs, and various socio-political influencers. These influencers include ethical issues surrounding patient care, social and political pressure from patients, their families, and non-governmental organizations (NGOs), among others (1, 3, 4).
Based on information from Iran’s news agencies regarding the current status of SMA medications in the country, two medicines, risdiplam and Spinraza, were introduced into Iran’s health system for the first time in 2022 by order of the president. These medicines are distributed free of charge to patients by the Ministry of Health and Medical Education. According to reports, the budget allocated for these two medicines is separate from the current budget of the Ministry of Health and Medical Education. The Program and Budget Organization, following the president's directive, will provide subsidies for these medicines to ensure patients do not have to worry about their high cost (6).
Spinal muscular atrophy is a costly disease due to its chronic nature and multidisciplinary care requirements. These costs can become a key barrier that limits patients’ access to life-saving care. Consequently, SMA patients heavily rely on governmental support and insurance coverage plans to afford their care, meet their healthcare needs, and maintain a quality life (1, 14).
The decision regarding the reimbursement of such medical drugs should not focus solely on their cost-effectiveness but rather on creating access to essential care and meeting patient needs. Despite the fact that none of the identified treatments were found to be cost-effective, they were covered and reimbursed by all countries (mostly high-income countries) through MEAs nonetheless. Managed entry agreements are a flexible tool that can help policymakers negotiate for the best outcomes, reduce care prices, and thus lower the overall cost of care for these patients and the financial burden on the healthcare system. These agreements can take various forms, and the specific pros and cons may vary depending on the type of MEA. Financial-based MEAs: These provide financial risk-sharing between the healthcare provider and the pharmaceutical company and may require extensive data collection and monitoring to ensure compliance.
Outcome-based MEAs: These link reimbursement to the actual clinical outcomes achieved by patients and require robust data collection and monitoring systems to track patient outcomes.
Performance-based MEAs: These align reimbursement with the real-world performance of the medical technology and require extensive data collection and monitoring to assess performance.
Access-based MEAs: These facilitate patient access to new, innovative treatments and may require additional administrative processes and infrastructure to manage patient eligibility and access (13, 15, 16).
It's important to note that selecting the type of MEA will depend on the healthcare system, the nature of the medical technology, and the specific terms and conditions of the agreement. It is suggested to conduct a study on selecting MEA models for the evaluation of orphan medicines in Iran.
Another approach to reducing the financial burden of this disease could be to invest in universal prevention and early diagnostics programs. Early screening for such abnormalities in patients’ DNA enables them to seek treatment in the earlier stages of the disease, before symptom onset. This, in turn, greatly reduces the overall treatment duration and its associated costs and burdens on both families and healthcare systems.
Early diagnosis of SMA through newborn screening programs can significantly improve patient outcomes and reduce healthcare costs. A study published in the Journal of Neuromuscular Diseases found that early detection of SMA through newborn screening allows for the timely initiation of disease-modifying therapies, which can lead to better motor function, reduced need for ventilatory support, and improved survival rates compared to late-diagnosed patients. Additionally, the study estimated that the implementation of universal newborn screening for SMA could result in substantial cost savings for healthcare systems, potentially offsetting the costs of screening and treatment over the lifetime of affected individuals. Early diagnosis and intervention are crucial in SMA, as they can significantly improve the quality of life and long-term outcomes for patients (17).
4.1. Limitations
Conducting a full HTA typically requires significant time and access to comprehensive data on the technology being evaluated. In contrast, this rapid HTA was undertaken due to the urgent need for policy decisions regarding the technology. As a result, the analysis relied primarily on secondary reports and clinical trial data, without the opportunity for critical appraisal of the included studies. This may have led to the inclusion of some lower-quality evidence. Additionally, the domestic-specific unit costs and budget impact analysis of the technology were not calculated as part of this rapid assessment. These limitations should be considered when interpreting the findings and recommendations from this rapid HTA process.