1. Background
Brain tumors are the most common solid tumors in childhood. These tumors can occur at any age and are slightly more common in males. The incidence of pediatric brain tumors varies among different countries. The annual incidence of brain tumors in children is approximately 6 per 100,000, resulting in around 600 new cases in Turkey, 3,700 new cases in the USA, and about 30,000 new cases worldwide each year (1, 2).
Brain tumors are responsible for a substantial rate of mortality and morbidity in childhood and are the leading cause of cancer-related mortality in children. The five-year overall survival rate is approximately 75% for children younger than 15 years old (2, 3). Gliomas are the most common type of pediatric brain tumors, while embryonal tumors (medulloblastoma) are the most common malignant tumors in this population (1).
The role of environmental factors is not clearly demonstrated, but radiation exposure has been associated with brain tumors in children (4, 5). Most pediatric brain tumors are influenced by genetic mutations, including NF, TSC, TERC, TP53, AKT3, and TERT (6). Interestingly, allergic conditions such as asthma, atopic conditions, and eczema have been shown to act as protective factors against the development of pediatric brain tumors (5-7).
Symptoms and signs are related to tumor localization, mass effect, tumor growth rate, and the age of the child. Additionally, tumor localization can vary according to age group. Supratentorial tumors are more common in infants and children older than 10 years, whereas infratentorial tumors are mostly seen in children between 4 and 10 years of age (8). There is no definitive correlation between symptoms and tumor type (9). The clinical presentation primarily depends on tumor localization and the age of the child. For instance, infants usually present with nonspecific symptoms such as irritability, vomiting, macrocephaly, and growth and developmental delays. In older children, focal symptoms may be more discernible due to increased intracranial pressure and focal nerve deficits. These symptoms can include seizures, headaches, nausea and vomiting, motor and sensory deficits, gait disorders, speech disorders, and impaired vision (8, 9).
The classification of pediatric brain tumors was last modified by the World Health Organization (WHO) in 2021 (10). Molecular mutations and aberrations were incorporated into the definitions, and new tumor types/subtypes were introduced based on DNA methylome profiling. With this new nomenclature, high-grade glioma now includes three new types in addition to DMG H3K27-altered: Diffuse hemispheric glioma H3 G34-mutant, diffuse pediatric-type high-grade glioma H3-wildtype and IDH-wildtype, and infant-type hemispheric glioma. The term "glioblastoma" has been revoked in pediatric-type neoplasms. Ependymoma is now divided into supratentorial (ZFTA fusion-positive or YAP1 fusion-positive), posterior fossa (group PFA or group PFB), and spinal ependymoma (MYCN-amplified) groups, while myxopapillary and subependymoma categories remain unchanged.
The main treatment options are surgical resection (gross total or subtotal), chemotherapy, and radiotherapy for patients older than 2 years. With advancements in diagnostic, imaging, and genetic tools, significant progress has been made in both the diagnosis and treatment of brain tumors. Molecular genetic alterations are now targeted for individualized therapy. Targeted therapies play a substantial role, particularly in brainstem tumors like DMG, which are not amenable to surgical resection due to their sensitive location. The most well-known medications include Nimotuzumab, an anti-EGFR (epidermal growth factor receptor) monoclonal antibody, and Ribociclib, a CDK4/6 inhibitor, both of which have shown potential effectiveness in treating DMG (11, 12).
Congenital brain tumors generally have a poor prognosis, with survival rates below 30%. Key factors that significantly affect survival include the tumor's malignant histology, size and location, stage of fetal development at diagnosis, and complications arising from treatment. From a general perspective, survival rates for childhood brain and spinal cord tumors vary depending on the specific tumor type. Additionally, survival rates can differ based on the tumor's grade or risk group, with lower-grade tumors generally associated with better outcomes (13, 14).
Studies presenting single-center experiences in brain tumors are valuable for several reasons. They provide detailed, context-specific data that can reveal unique local challenges, practices, and outcomes that may not be captured in multi-center studies. These insights are crucial for understanding the nuances of treatment approaches and patient management in a specific healthcare setting. Additionally, single-center studies can demonstrate the impact of institutional resources and patient demographics on treatment efficacy and outcomes, providing essential information for developing tailored strategies and improving practices in specific settings.
The treatment of brain tumors requires a multidisciplinary approach involving neurosurgery, pediatric oncology, radiation oncology, neurology, endocrinology, pediatric intensive care, and palliative care. In centers with such a comprehensive care model, patient outcomes are significantly improved. Our university hospital, located in Istanbul, where a large portion of the population resides, is a leading center for pediatric oncology in the country. The healthcare system in Turkey is organized in a stepwise manner. Patients suspected of having brain tumors in primary care centers are referred to our tertiary center.
2. Objectives
The objective of this study is to examine the clinical and epidemiological characteristics of pediatric brain tumor cases over a 5-year period. Additionally, the study aims to evaluate long-term side effects and identify areas for improvement, including increasing surgical resection rates, expanding the use of molecular profiling, and integrating palliative care to enhance patient outcomes.
3. Methods
This retrospective study included children with brain tumors treated at Istanbul Medical Faculty Pediatric Oncology Clinic between 2018 and 2023. Admission symptoms, age at diagnosis, presenting findings, treatment modalities (surgery, radiotherapy, and chemotherapy), treatment responses, and side effects were reviewed retrospectively.
The 2016 and 2021 WHO classifications of CNS tumors were used to classify the patients. Pediatric CNS tumors were primarily divided into two groups: Glial tumors and non-glial tumors. Glial tumors include gliomas, glioneuronal tumors, and ependymal tumors, while non-glial tumors include embryonal tumors, germ cell tumors, choroid plexus tumors, and craniopharyngiomas. Protocols were followed as per the recommendations in *Lanzkowsky's Manual of Pediatric Hematology and Oncology*. Patients were evaluated during treatment and after the cessation of treatment to assess long-term side effects.
The inclusion criteria for this study encompassed pediatric patients under 18 years of age who were diagnosed with brain tumors, confirmed through central pathological and radiological review. Patients were required to have been treated and followed up within the specified clinic between 2018 and 2023, with complete clinical, radiological, and pathological data available. Additionally, they must have undergone at least one form of treatment, such as surgery, chemotherapy, or radiotherapy. The exclusion criteria included patients older than 18 years, those with incomplete or missing medical records, patients with non-primary brain tumors (e.g., metastases from other cancers), individuals who did not receive treatment or were lost to follow-up, and those with significant medical conditions that could interfere with the study outcomes.
The diagnosis of brain tumors was typically established through a combination of clinical evaluation, imaging studies, and histopathological examination. The initial assessment often involved neuroimaging techniques such as magnetic resonance imaging (MRI) or computed tomography (CT) scans to identify the presence, location, and characteristics of the tumor. Following imaging, a definitive diagnosis usually required histopathological analysis of tumor samples obtained through biopsy or surgical resection. Additionally, molecular and genetic testing were utilized to provide further diagnostic and prognostic information.
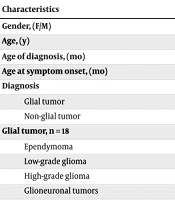
A total of 30 patients were included in this study. Fourteen of them (47%) were male, and 16 (53%) were female. The mean age of the children was 8.8 ± 3.1 years, the mean age at diagnosis was 65.6 ± 37.7 months, and the mean age of symptom onset was 61.8 ± 36.8 months. Four patients (13%) were asymptomatic. The most common symptom was vomiting (40%), followed by visual disturbances (blurred vision, strabismus, gaze limitation) (37%), headache (33%), gait disturbances (30%), and psychological problems (sleepiness, forgetfulness) (7%). The mean time between symptom onset and diagnosis was 3.76 months, with a median of 1 month. The mean follow-up duration was 31 months, with a median of 24 months (range: 12 - 60 months).
In the follow-up assessment, we classified the response of patients with brain tumors into four categories: Progressive disease, partial response, complete response, and stable disease. Complete response required all of the following criteria: Complete disappearance of all enhancing measurable and non-measurable disease sustained for at least 4 weeks; no new lesions; and stable or improved non-enhancing (T2/FLAIR) lesions. Additionally, patients had to be off corticosteroids (or on physiologic replacement doses only) and remain clinically stable or improved (15).
Partial response required the following criteria: A 50% decrease compared with baseline in the sum of the products of perpendicular diameters of all measurable enhancing lesions, sustained for at least 4 weeks; no progression of non-measurable disease; no new lesions; and stable or improved non-enhancing (T2/FLAIR) lesions on the same or a lower dose of corticosteroids compared with the baseline scan. The patient also had to be on a corticosteroid dose not greater than the dose at the time of the baseline scan, with stable or improved clinical status (15).
Stable disease was observed if the patient did not meet the criteria for complete response, partial response, or progression, and required the following conditions: Stable non-enhancing (T2/FLAIR) lesions on the same or a lower dose of corticosteroids compared with the baseline scan, and clinically stable status (15).
Progression was defined by any of the following criteria: A 25% or greater increase in the sum of the products of perpendicular diameters of enhancing lesions (compared with baseline if no decrease) on stable or increasing doses of corticosteroids; a significant increase in T2/FLAIR non-enhancing lesions on stable or increasing doses of corticosteroids compared with the baseline scan or the best response after initiation of therapy, not due to comorbid events; the appearance of any new lesions; clear progression of non-measurable lesions; or definite clinical deterioration not attributable to other causes apart from the tumor or to a decrease in the corticosteroid dose (15).
Patient outcomes were defined using the terms mild, moderate, and severe neurological deficits. In the assessment of our patients' motor functions, the gross motor function classification system (GMFCS) criteria were utilized. Mild neurological deficit was defined as slight impairments in neurological function that caused minimal disruption to daily activities. These deficits were characterized by subtle symptoms, such as minor weakness, mild sensory changes, or slight coordination difficulties, which did not significantly affect the patient's overall quality of life. Moderate neurological deficits were characterized by more pronounced impairments that noticeably affected a patient's ability to perform daily tasks. Symptoms included moderate weakness, sensory loss, coordination difficulties, or cognitive challenges, which necessitated some level of assistance or adaptation in daily living. Severe neurological deficits, on the other hand, involved significant impairments that severely restricted a patient's independence and ability to carry out basic daily activities. These included profound weakness or paralysis, substantial sensory loss, severe cognitive impairments, or other disabling conditions that required continuous care and support (16). Patients were evaluated both during treatment and after its cessation in terms of long-term outcomes.
A limitation of the study is the small sample size, which affects its generalizability. Hopefully, future collaborative studies will analyze and provide further insights into the prognosis and side effects of childhood brain tumors.
3.1. Statistics
The SPSS 23.0 (IBM Corp) program was used for statistical analysis. Descriptive analysis of quantitative variables was performed. For the evaluation of categorical variables, the chi-square test was conducted. A P-value of < 0.05 was considered significant.
4. Results
4.1. Tumor Classification
Thirty children were diagnosed with brain tumors, treated, and followed up in our clinic. Among them, 18 (60%) had glial tumors and 12 (40%) had non-glial tumors. Among the glial tumors (n = 18), 11 patients had glioma (4 low-grade gliomas, 7 high-grade gliomas), 5 patients had ependymoma (4 posterior fossa and 1 supratentorial), and 2 patients had glioneuronal tumors.
Within the high-grade gliomas, 4 patients had diffuse midline glioma (DMG) H3K27-altered, and 3 patients had diffuse pediatric-type high-grade glioma H3-IDH wildtype. Among the non-glial tumors (n = 12), 10 patients had embryonal tumors (8 medulloblastomas, 2 ATRT) and 2 patients had germinomas (Table 1). Within the medulloblastoma patients, 1 patient had WNT, 1 had SHH, and 5 had non-WNT/non-SHH subtypes. At the time of diagnosis, 3 patients (10%) had metastasis to the spine.
| Characteristics | Values a |
|---|---|
| Gender (F/M) | 17/14 |
| Age (y) | 8.8 ± 3.1 |
| Age of diagnosis (mo) | 65.3 ± 37.7 |
| Age at symptom onset (mo) | 61.8 ± 36.8 |
| Diagnosis | |
| Glial tumor | 18 (60) |
| Non-glial tumor | 12 (40) |
| Glial tumor, n = 18 | |
| Ependymoma | 5 (27) |
| Low-grade glioma | 4 (22) |
| High-grade glioma | 7 (39) |
| Glioneuronal tumors | 2 (12) |
| Non-glial tumor, n = 12 | |
| Medulloblastoma | 8 (66) |
| ATRT | 2 (17) |
| Germinoma | 2 (17) |
| Presenting symptoms | |
| Vomiting | 40 |
| Visual disturbance | 37 |
| Headache | 33 |
| Gait disturbance | 30 |
a Values are expressed as mean ± SD or No. (%).
3.2. Treatment Modalities
Surgery was performed as gross total resection (GTR) or subtotal/partial resection (STR). Gross total resection was performed in 8 patients (27%), and STR was performed in 11 patients (37%). Eleven patients (36%) did not undergo surgery (Table 2).
| Characteristics | Percentage |
|---|---|
| Surgery technics | |
| Not done | 36 |
| STR | 37 |
| GTR | 27 |
| Chemotherapy | |
| Yes | 77 |
| No | 23 |
| Radiotherapy | |
| Yes | 57 |
| No | 43 |
| Treatment outcome | |
| Responsive (complete/partial) | 57 |
| Stable | 30 |
| Progressive | 13 |
| Neurological deficit | |
| No/mild | 73 |
| Moderate/severe | 27 |
| Status | |
| Deceased | 13 |
| Alive | 87 |
Abbreviations: GTR, gross total resection; STR, subtotal resection
Chemotherapy was administered either intravenously or orally. A total of 23 patients (77%) received chemotherapy. Vinca alkaloids, particularly vincristine, were the mainstay of therapy. Temozolomide (n = 7, 23%) was the most commonly used oral medication. Platinum agents (Cisplatin/Carboplatin) were used in nearly half of the patients (n = 13, 45%). Alkylating agents (Cyclophosphamide, Ifosfamide) were used in one-third of patients (n = 10, 35%). Bevacizumab was administered to 5 patients (16%), and 1 patient received Nimotuzumab.
Seventeen patients (57%) received cranial or cranio-spinal irradiation, of which 6 patients (35%) received cranial irradiation and 11 patients (65%) received cranio-spinal irradiation.
4.3. Therapy Responses
Fifty-seven percent of the patients responded to treatment (complete or partial response), 30% had stable disease, and 13% had progressive disease.
4.3.1. Long-term Side Effects
While 73% of our 26 patients had no or mild neurological deficits, 27% experienced moderate to severe disabilities (including gait impairment, hearing impairment, visual impairment, and speech disturbances).
4.3.2. Survival
Four patients passed away (2 with high-grade glioma and 2 with embryonal tumors), and 26 patients are alive. The overall mortality rate was 13%.
5. Discussion
In this study, we aimed to present primary brain tumors treated at our center. The mean time between the onset of symptoms and diagnosis was 3.8 months, with a median of 1 month. Of the patients, 57% had a complete or partial response to treatment, and 13% had disease progression. The overall mortality rate was 13%. Among survivors, nearly one-fourth had moderate to severe neurological sequelae, and more than one-third of the patients did not undergo surgery. As observed, short- and long-term neurological sequelae in brain tumors can significantly impact a patient's quality of life.
Common symptoms included persistent headaches, nausea, and vomiting due to brain irritation or elevated intracranial pressure, and seizures resulting from abnormal brain activity during treatment. Additionally, motor impairments such as weakness or paralysis occurred if tumors affected motor areas, while sensory deficits, visual disturbances, and speech disorders arose if sensory pathways, optic nerves, or speech centers were involved. Coordination, cognitive, and behavioral changes were observed if tumors impacted the prefrontal cortex or other cognitive areas. The severity and type of symptoms depended on the tumor's type, location, and size. Symptoms could also be secondarily aggravated by sequelae from surgery, radiation, chemotherapy, or disease progression.
We acknowledge that certain neurological symptoms may indeed be induced by medications and could be transient. The moderate to severe neurological sequelae reported among survivors were assessed at follow-up after the acute treatment phase and were considered long-term outcomes, not directly attributable to temporary medication effects. These sequelae were persistent and significantly impacted the patients' quality of life, qualifying as long-term consequences of the disease and its treatment.
Symptoms and complaints are primarily related to tumor localization and its secondary effects. In our sample, 70% of patients had infratentorial tumors. The most common symptom was vomiting (40%), followed by visual disturbances (37%), headaches (33%), gait disturbances (30%), and psychological problems (7%). These findings were similar to those of a recent study indicating vomiting (40%), headaches (47%), and vision problems (20%) (17). The mean time between the onset of symptoms and diagnosis was 3.76 months, with a median of 1 month. A median 1-month duration is an acceptable time frame when compared to the literature, which cites a diagnosis interval of 1 month (18, 19), 1.5 months (20-22), and 2 months (23, 24). Given the extended mean pre-diagnosis interval, greater emphasis should be placed on parent awareness and the inclusion of symptomatology in medical education curricula.
Tumor surgery is crucial for therapy, as the extent of resection remains the most important factor for almost all types of brain tumors (25). Tissue samples are essential for pathological diagnosis and for determining the molecular characteristics of the tumor. In our limited sample-sized study, more than one-third of patients were unable to undergo surgery. Non-surgical patients included both those with low-grade glioma and high-grade glioma. To achieve better outcomes and improve survival rates, the resectability rate should be increased in eligible patients. In cases where surgery is not an option, at least a biopsy should be performed to allow for molecular testing, enabling targeted therapy for treatment.
Herdell et al. showed in their multi-center studies that the extent of neurosurgical treatment significantly impacts overall survival, and the extent of surgery can even influence prognosis (26). Mitchell et al. emphasized in their article that maximal tumor resection improves both overall survival and quality of life. However, the extent of maximal surgical resection should be balanced to avoid causing neurological sequelae. Hence, the concept of "maximal safe resection" is a key principle in our field (27). On the other hand, surgical complications and long-term neurological sequelae should be closely monitored to maintain the quality of life for patients. Pituitary dysfunction, stroke, intracranial hemorrhage, seizures, hemiparesis, visual impairment, and gait impairment are some of the neurological complications that can arise from surgery (28).
Temozolomide is an oral alkylating agent. Its most notable features include rapid absorption after oral administration, very high bioavailability, and efficient crossing of the blood-brain barrier (BBB) (100%) (29). The main mechanism of action of TMZ involves the addition of a methyl group to DNA. This methylated DNA (especially methylguanine) acts as a carcinogen and mutagen, bypassing the DNA mismatch repair (MMR) mechanism. As a result, DNA damage occurs, leading to cell death and apoptosis (30). The counteracting mechanism for methylation is the cell's innate demethylation process, facilitated by methylguanine DNA methyltransferase (MGMT). Reduced MGMT expression renders cells more susceptible to TMZ, thereby making the treatment more effective. Therefore, the methylation status of tumor cells is crucial in determining prognosis (31). In glioma patients, TMZ has been commonly used for many years. Unfortunately, we were unable to determine the methylation status of our patients. Following this study, we will standardize the process of checking the methylation ratio in glioma patients.
Attention to long-term effects has increased as survival rates among children with cancer have significantly improved. Overall survival of childhood cancer has risen from 30% to nearly 80% over the past half-century (32). Cancer survivors are at risk for long-term side effects, including neurological, cardiological, endocrinological, psychological, and socioeconomic sequelae (33).
Several population-based studies have investigated long-term organ involvement among survivors and found a 5-fold increased risk of endocrinopathy (34) and an 8.5-fold increased risk of stroke (35). In another study, 20% of patients developed significant hearing loss after a Cisplatin-based regimen (36). Secondary neoplasms are also a major concern among survivors. Studies have revealed that survivors are at a 22-fold increased risk for primary bone cancer and a 30-fold increased risk for soft tissue sarcoma (37, 38). In our study, one in four patients had moderate to severe neurological sequelae. Although our limited sample size did not allow us to draw broader conclusions, this ratio (1/4) is consistent with the literature. However, we did not detect any secondary cancers among the survivors.
The limitations of this study include the small sample size (a total of 30 patients), which restricts its generalizability, as well as its design as a single-center retrospective study. Additionally, the limited availability of long-term follow-up data is due to the relatively short follow-up periods for the patients. As we do not have a dedicated palliative care unit, long-term follow-up and management of sequelae (neurological, endocrinological, etc.) may have been interrupted. This study primarily highlights the urgent need for a long-term follow-up clinic and a multidisciplinary approach for children with brain tumors.
5.1. Conclusions
In this study, we evaluated the characteristics and treatment outcomes of pediatric brain tumors. While our limited sample size restricts the generalizability of the findings, the study has shown that surgery options should be expanded, or at least a biopsy should be performed in severe cases to enable molecular profiling. Methylation status should be assessed, especially in high-grade gliomas. For long-term side effects and the quality of life of patients, the palliative care team should be involved from the time of diagnosis. A holistic approach is crucial in managing pediatric brain tumor cases.