1. Background
Nucleated red blood cells (nRBCs) are rarely present in the circulation of healthy children and adolescents because the nucleus of the developing normoblast is typically extruded before the cell exits the bone marrow. This process is so efficient that the presence of nRBCs in circulation raises concerns about hemopathology. However, nRBCs are frequently observed in cases of intrauterine growth restriction (IUGR), neonates with fetal anomalies, and premature infants (1-3). Hypoxia is hypothesized to be a significant factor that increases the number of circulating nRBCs, either through stimulated erythropoiesis (4-6) or by depleting storage pools. Several studies have demonstrated an association between elevated nRBC levels and poor perinatal outcomes, including necrotizing enterocolitis (NEC) (7, 8), severe intraventricular hemorrhage (IVH) (9, 10), periventricular leukomalacia (PVL) (11, 12), and mortality (13). Evidence regarding the relationship between nRBC count and the incidence of retinopathy of prematurity (ROP) is conflicting. Lubetzky et al. and Christensen et al. reported an association between high nRBC counts and ROP incidence (14, 15), while Gotru et al. did not confirm this association (16).
2. Objectives
In this study, we evaluated the association between premature mortality and severe morbidity in premature infants.
3. Methods
In this cohort study, the statistical population comprised premature infants admitted to the neonatal intensive care unit (NICU) at Taleghani Hospital in Ilam City from February 2019 to March 2020. Infants with anomalies and those whose mothers did not consent to a complete blood count (CBC) test on days 1 and 5 were excluded. A total of 450 premature infants were admitted to the NICU during this period using a simple and available sampling method. Morbidities, epidemiologic data, and outcome parameters were extracted from electronic records. Survival was defined as living until discharge or transfer to another hospital. Premature infants were categorized into early and late preterm groups, defined as less than 34 weeks and 34 to 36 weeks and 6 days, respectively. The Apgar score ranged from 0 to 10. Infants requiring ventilation were included in the study.
Initial data, including birth date, gender, weight, gestational age (GA), and corticosteroid intake prior to delivery, were collected using a pre-prepared checklist. Consent forms were obtained from mothers for CBC testing on days 1 and 5. The nRBC count of preterm infants was evaluated and recorded on these days. The fifth day was chosen because nRBC levels are typically high in healthy infants on the first day but not on the fifth day unless there are complications. For this purpose, 2 mL of blood was collected and sent to the laboratory in an EDTA-containing tube. The sample was assessed using a cell counter device. A drop of the blood sample was placed on a slide, fixed with methanol, stained using Giemsa stain, and examined under a microscope at 100x magnification.
Infants were monitored for prematurity complications, such as ROP, bronchopulmonary dysplasia (BPD), NEC, IVH, and mortality rate, during their hospital stay and up to GA = 40 weeks. They were divided into death and survival groups, and relevant data were collected and recorded in a checklist. The time from symptom onset to recovery or death was considered the survival time. Univariate data analysis was performed using the best distribution with the lowest Akaike information criterion (AIC). A P-value of less than 0.2 was considered statistically significant in univariate analysis. All significant variables were entered into a multivariate model using the accelerated failure time (AFT) distribution based on the lowest AIC. Accelerated failure time models use the median survival ratio [time ratio (TR)] instead of the hazard ratio. Data were analyzed using STATA software version 12 (StataCorp, College Station, TX).
4. Results
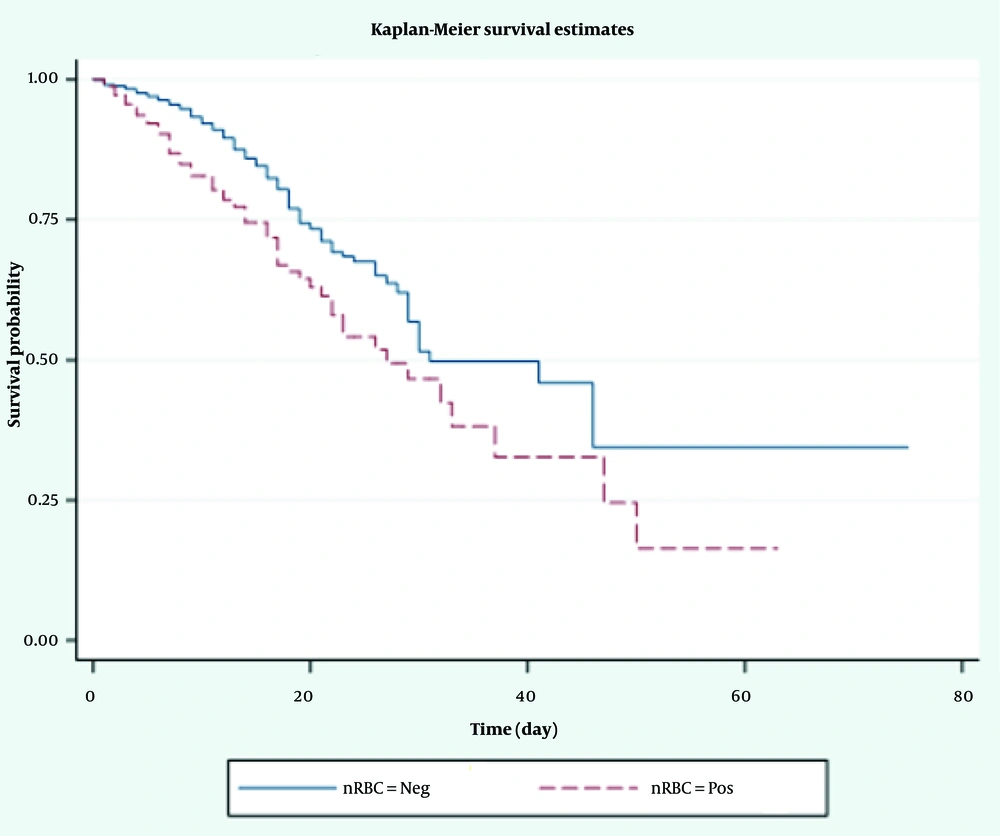
A total of 450 premature infants were included in the data registry. The mean weights of deceased and surviving infants were 2197.92 ± 564.94 g and 2349.03 ± 536.65 g, respectively. The mean nRBC levels were 37.93 ± 12.03/100 WBC in deceased infants and 39.52 ± 11.05/100 WBC in surviving infants. Nucleated red blood cells were present in 54.8% of deceased patients and 45.2% of surviving patients. The total follow-up days for all infants were 4361 days, with 3222 days for deceased patients and 1139 days for surviving patients. According to the Kaplan-Meier curve, the median survival times were 9 days for nRBC-positive patients and 11 days for nRBC-negative patients (Figure 1). Descriptive statistics for other patients are presented in Table 1.
| Variables | Survivors (n = 357) | Deceased (n = 93) | Cox Proportional Hazard Ratio (95% CI) | P-Value |
|---|---|---|---|---|
| Gender (female) | 187 (50.7) | 43 (43.9) | 1.47 (0.84 - 2.56) | 0.043 |
| Birth weight (g) | 2349.03 ± 536.65 | 2197.92 ± 564.94 | 1.04 (1.03 - 1.04) | < 0.001 |
| GA | ||||
| 34 - 36 (wk) and 6 (d) | 34.10 ± 1.95 | 33.41 ± 3.68 | 1.50 (1.18 - 1.84) | < 0.001 |
| Past medical history of the mother during pregnancy | ||||
| Pregnancy blood pressure | 53 (14.8) | 8 (8.6) | 1.73 (1.38 - 2.18) | < 0.001 |
| Preeclampsia | 20 (11.1) | 4 (2.3) | 0.98 (0.96 - 1.01) | 0.06 |
| Gestational diabetes | - | - | - | |
| Without disease | 284 (71.4) | 81 (89.1) | 0.70 (0.66 - 0.81) | 0.07 |
| Mother's history of steroid intake | ||||
| Positive | 108 (30.3) | 32 (34.4) | 0.98 (0.96 - 1.01) | 0.06 |
| ROP | 147 (41.2) | 38 (40.9) | 1.57 (1.26 - 1.96) | < 0.001 |
| BPD | 14 (3.9) | 4 (4.3) | 1.75 (1.00 - 3.05) | 0.53 |
| NEC | 2 (0.6) | 0 (0.0) | 2.26 (1.62 - 3.15) | 0.62 |
| IVH | 2 (0.6) | 3 (3.2) | 1.94 (1.35 - 2.78) | 0.06 |
| nRBC | 39.52 ± 11.05 | 37.93 ± 12.03 | 0.89 (0.85 - 0.94) | < 0.001 |
Gender Distribution, Birth Weight, a History of Steroid Intake by the Mother, and a Past Medical History of the Mother a
Univariate analysis using the logarithmic distribution confirmed a significant association between nRBC levels and mortality, with a TR of 1.07 (95% CI: 1.05 - 1.17; P < 0.001), indicating an increased risk of mortality with rising nRBC levels (Table 2).
| Variables | TR (95% CI) a | P-Value |
|---|---|---|
| Gender | ||
| Male | 1 b | |
| Female | 0.66 (0.56 - 0.79) | 0.06 |
| Birth weight (g) | 0.79 (0.53 - 1.07) | 0.41 |
| GA | 0.53 (0.28 - 1.05) | 0.08 |
| Past medical history of the mother during pregnancy | 0.63 (0.50 - 0.98) | < 0.001 c |
| Mother's history of steroid intake | 0.66 (0.51 - 0.85) | < 0.001 c |
| BPD | 0.59 (0.47 - 0.78) | < 0.001 c |
| NEC | 0.67 (0.66 - 0.89) | 0.07 |
| IVH | 0.70 (0.48 - 0.99) | 0.04 c |
| nRBC | 1.07 (1.05 - 1.17) | < 0.001 c |
Univariate Accelerated Failure Time Regression Model Analysis of Nucleated Red Blood Cells Levels and the Other Factors for Mortality in Premature Infants
In the multivariate model adjusted for other variables, the adjusted TR for nRBC level was 1.05 (95% CI: 1.01 - 1.08; P = 0.04; Table 3).
| Variables | Adjusted TR (95% CI) | P-Value |
|---|---|---|
| Gender | ||
| Male | 1 b | 0.06 |
| Female | 0.62 (0.51 - 0.89) | |
| Birth weight (g) | 0.83 (0.61 - 1.15) | 0.04 c |
| GA | 0.60 (0.57 - 1.69) | 0.94 |
| Past medical history of the mother during pregnancy | 0.69 (0.51 - 1.19) | 0.28 |
| Mother's history of steroid intake | 0.71 (0.70 - 1.01) | 0.91 |
| BPD | 0.61 (0.50 - 1.04) | 0.04 |
| NEC | 0.70 (0.59 - 0.99) | 0.08 |
| IVH | 0.73 (0.58 - 1.19) | 0.05 |
| nRBC | 1.05 (1.01 - 1.08) | 0.04 c |
Multivariate Analysis Using Weibull the Accelerated Failure Time Model for the Effect of Nucleated Red Blood Cells Levels on the Mortality of Premature Infants a
5. Discussion
This study demonstrated that the nRBC count could serve as a marker for mortality in premature infants. The analysis revealed no significant differences in mortality rates between the two groups regarding gender and GA, although differences in weight were observed. Morbidities such as BPD, ROP, and IVH were significantly different between the two groups (P < 0.05), whereas no differences were found in NEC. As previously mentioned, fetal distress and tissue hypoxia stimulate erythropoietin production, which regulates erythropoiesis and accelerates the release of less differentiated RBCs into extramedullary regions (17). Poryo et al. found a significant association between increased nRBC counts and severe IVH, NEC, and perinatal mortality (18). Our study's findings regarding the association between nRBCs and IVH and perinatal mortality align with these results. However, the association between nRBCs and NEC was not significant in our study. Poryo et al. observed that nRBC counts and lactate levels on the second day after birth were associated with infant mortality (19). A 2023 study showed that increased nRBC counts on days 2 and 5 of birth were independent predictive factors for death in premature infants (20). Conversely, Loesaus et al. studied 176 preterm infants and examined complications such as IVH, BPD, and NEC in relation to nRBC counts. They found that increased nRBC counts on day 3 of birth were independent predictive factors for complications, consistent with our findings regarding BPD and IVH. However, no association was observed between increased nRBC counts and NEC in our study, likely due to differences in sample size (21).
Since previous studies have not examined the association between increasing nRBC counts and mortality concerning infants' weight, gender, and GA, the present study could be a promising step in identifying this association. If it is observed that mortality rates increase in low-birth-weight infants with higher nRBC counts, it may underscore the importance of studying nRBC counts in these infants. Ultimately, our study results indicated that the number of nRBCs in the peripheral blood of premature infants is indicative of intrauterine hypoxia (22). Thus, it could be used as a prognostic factor to predict mortality and morbidity in premature infants.
5.1. Conclusions
The presence of nRBCs may indicate fetal hypoxia. Given the significant association between increased nRBC counts and the mortality of low-weight infants, as well as morbidities such as ROP, IVH, and BPD, nRBCs could serve as an inexpensive and readily available prognostic factor for mortality in premature infants.
5.2. Limitations
This study has limitations due to the small sample size and variability in nRBC counts. Additionally, the analyzer may not detect old, depleted samples or samples with nRBC counts below 200/μL, potentially leading to false-negative results. A high white blood cell count can also adversely affect the results.