1. Background
According to the WHO, preterm birth (PTB) is defined as a birth that occurs before 37 weeks of gestation (259 days) (1). The prevalence of preterm labor ranges from 5% to 18%, with low-and middle-income countries accounting for approximately 60% of preterm births worldwide (2). In China, the average preterm birth incidence is 7.1% (3). Preterm birth has significant implications for neurodevelopmental functioning and long - term health, including an increased risk of cerebral palsy, learning disabilities, and chronic diseases in adulthood (4). The pathogenesis of PTB is not fully understood and may be related to genetic factors, environmental factors, maternal nutrition, and other variables (5). Maternal nutritional status during pregnancy is an important modifiable factor that affects the mother's health and influences the growth and development of the next generation (6). Adequate nutrition is particularly crucial for the growth and development of infants and the maintenance of normal physiological functions, and it primarily comes from the diet (7). Micronutrient deficiencies occur when there are insufficient reserves in the body before pregnancy and inadequate supplies after pregnancy (8). Copper is an essential trace element and is most abundant in the brain and liver (9). Copper is involved in many metabolic processes in the body, and copper - based enzymes play a role in iron metabolism as cofactors, which can help prevent miscarriage and anemia during pregnancy (3). Zinc (Zn) is the second most abundant trace element in the human body after iron (10). In cells, zinc exists as a divalent ion (Zn2+) (11). Skeletal muscle has the highest levels of zinc (12). Zinc has many roles in the body, including immune function, growth and development, neurological function, vision, and fertility (12). Some studies have shown that zinc supplementation during pregnancy reduces the risk of preterm birth (13). In recent years, there has been extensive scientific interest in the biological effects of various micronutrients during pregnancy and their relationship to the origins of life, genetics, growth and development, congenital disabilities, and other maternal and child health outcomes (8). However, existing studies on the relationship between copper or zinc intake during pregnancy and preterm labor have not systematically covered the preconception period and the entire pregnancy (14-22). Some studies that have assessed total copper/zinc intake have not separately modeled the relationship between copper/zinc intake from food and copper/zinc intake from dietary supplements. Additionally, previous studies have not considered the synergistic effect of copper and zinc intake on the risk of preterm labor.
2. Objectives
The aim of the present study was to investigate the effect of daily copper and zinc intake before and during pregnancy on the risk of labor and delivery, and to assess whether copper and zinc intake interact with the risk of preterm labor.
3. Methods
3.1. Study Population
A birth cohort study was conducted from January 2018 to June 2019 at the largest hospital of its kind in Lanzhou City, Gansu Province. Eligible study participants were pregnant women without a history of mental illness, who were 18 years of age or older, had regular prenatal care, and provided written informed consent. A total of 8,897 eligible women participated, including 880 who delivered preterm infants and 8,017 who delivered at term. This study was approved by the Institutional Review Board of Maternal and Child Health Hospital of Gansu Province [2018 (029)]. Participants provided written informed consent.
3.2. Dietary Surveys, Copper, and Zinc Daily Intake
Daily surveys and dietary intake of copper and zinc were collected using a face-to-face semi-quantitative food frequency questionnaire (FFQ) (23). The survey included sociodemographic characteristics, medical history, obstetric history, and dietary intake, covering 40 items (Appendix 1 in Supplementary File). Participants reported the types and quantities of various foods consumed over three consecutive days, encompassing 59 common foods in 12 categories: Cereals, fats and oils, vegetables, fruits, poultry, animal meat and its products, eggs, aquatic products, legumes and legume products, milk and milk products, mushrooms and algae, and snacks and beverages.
The questionnaires were administered during the first trimester (1 - 13 weeks), second trimester (14 - 27 weeks), and third trimester (> 27 weeks) of pregnancy. After completing all dietary surveys, the daily dietary intake of vitamins and micronutrients for each pregnant woman at different trimesters was calculated according to the second edition of the 2009 Chinese Food Composition Table (24). According to the Recommended Nutrient Intake (RNI) for Chinese residents (25), the daily intake of vitamins and micronutrients in different trimesters was categorized into groups for statistical analysis. The RNI is defined as the amount of nutrients sufficient to meet the needs of 97.5% of the population. Data on pregnancy-related complications and delivery outcomes were obtained from medical records.
3.3. Preterm Labor
Preterm labor (PTB) was defined as deliveries occurring at less than 37 weeks of gestation (259 days) and were included in the case group. Deliveries occurring at more than 37 weeks of gestation were defined as non–preterm (full-term births) and were included in the control group for analysis. Preterm births were further categorized by etiology into medically induced preterm births and spontaneous preterm births.
3.4. Covariates
The study covariates were as follows: (1) socio-demographic characteristics, including maternal age, ethnicity (Han/Minority), monthly income (< 3000 RMB per capita/≥ 3000 RMB per capita), education level (college or above/below college), smoking during pregnancy (passive and active), and employment status (No/Yes). Passive smoking was defined as exposure to another person's tobacco smoke for more than 15 minutes per day; (2) Maternal health - related factors, including multivitamin supplementation (No/Yes), anemia during the first trimester diagnosed by physicians using the criteria of hemoglobin concentration lower than 110 g/L, gestational diabetes (No/Yes), and gestational hypertension (No/Yes).
3.5. Statistical Analysis
Comparisons of the selected characteristics between women with preterm birth and term birth were evaluated using the chi-square test or Fisher's exact test. Differences in measurement data between the two groups were analyzed using an independent-sample t-test, and variables that did not follow a normal distribution were compared between groups using the Wilcoxon rank - sum test. Unconditional logistic regression was employed to determine the odds ratios (ORs) and 95% confidence intervals (CIs) for the association between dietary copper and zinc intake and the risk of preterm birth and its clinical subtypes. Confounding factors, including weight gain during pregnancy, father's BMI, total energy intake, monthly income per capita, maternal education level, smoking, maternal employment, multivitamin supplementation, gestational hypertension, history of premature birth, and reproductive history, were adjusted for in the unconditional logistic regression models. Dietary copper and zinc intake were categorized into quartiles, and the dose - response relationship (P for trend) was calculated based on those categorical levels. The association between dietary copper/zinc intake levels and the risk of preterm birth may result in a nonlinear correlation. Multivariate-adjusted restricted cubic spline (RCS) models with three knots were applied to explore this nonlinear association. The number of knots was determined by comparing the Bayesian and Akaike information criteria. The RCS models were adjusted for the potential confounding factors listed above. The multiplicative interaction parameters [OR = OR11 / (OR01 × OR10)] and 95% CI were also estimated by including the aforementioned variables. The interactions on the additive scale were assessed using relative excess risk due to interaction (RERI = RR11 − RR10 − RR01 + 1), attributable proportion (AP = RERI/RR11), and synergy Index [S = (RR11 − 1)/[ (RR01 − 1) + (RR10 − 1)]]. We estimated 95% CI for each of these measures; the null values for RERI and AP were 0, while the null value for S was 1. All statistical tests were two - sided. Analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). The RCS models were executed using R software, version 4.1.3 (packages 'Hmisc', 'rms', and 'survival').
4. Results
4.1. Basic Characteristics of the Study Population
The study population has been described earlier. As shown in (Appendices 1, 2, 3 in Supplementary File), mothers of preterm infants had lower monthly household incomes, lower educational attainment, lower employment rates, higher prevalence of gestational hypertension, higher prevalence of gestational diabetes, higher prevalence of smoking, and a history of preterm birth and childbearing compared to mothers of non-preterm infants. Additionally, case mothers were older, had a higher pre-pregnancy Body Mass Index, significantly lower weight gain, lower total energy intake, less frequent multivitamin supplementation, and lower dietary intake of copper and zinc. Ethnic minorities were at greater risk of preterm birth. There were no significant differences between the two groups of mothers in terms of alcohol consumption during pregnancy, anemia during pregnancy, or history of miscarriage.
4.2. Associations of Maternal Dietary Copper Intake with the Risk of Preter
As shown in Table 1, the adjusted ORs for preterm labor were 1.05 (0.96 - 1.14), 1.05 (0.93 - 1.18), and 1.04 (0.82 - 1.32) for preterm births when compared to the highest quartile of pre - pregnancy dietary copper intake (quartile 4), the highest quartile of intake (quartile 3), and the lowest quartile of intake (quartile 2) to the lowest quartile of intake (quartile 1), respectively. The test for trend was significant (P = 0.013). Copper intake in quartile 1, quartile 2, and quartile 3 was associated with a significantly increased risk of spontaneous preterm labor compared to the highest quartile, and the test for trend was significant (P = 0.0107). After stratification by trimester period, copper intake was not observed to be associated with an increased risk of preterm labor in the early and middle trimesters of pregnancy. However, copper intake during the last trimester of pregnancy was associated with an increased risk of preterm labor and spontaneous preterm labor.
| Dietary Copper Intake (mg/d) | Term (N = 8017) | Preterm (N = 880) | Medically Indicated | Spontaneous | ||||
|---|---|---|---|---|---|---|---|---|
| Cases | OR a (95% CI) | OR b (95% CI) | Cases | OR b (95% CI) | Cases | OR b (95% CI) | ||
| Before Pregnancy | ||||||||
| Q1 < 1.45 | 1926 | 299 | 1.23 (1.15 - 1.32) | 1.05 (0.96 - 1.14) | 99 | 0.97 (0.83 - 1.14) | 200 | 1.08 (0.97 - 1.19) |
| Q2 1.45 - 2.06 | 2005 | 218 | 1.14 (1.03 - 1.27) | 1.05 (0.93 - 1.18) | 68 | 1.00 (0.80 - 1.25) | 150 | 1.07 (0.93 - 1.23) |
| Q3 2.06 - 2.79 | 2032 | 192 | 1.14 (0.92 - 1.41) | 1.04 (0.82 - 1.32) | 55 | 0.91 (0.58 - 1.44) | 137 | 1.10 (0.84 - 1.45) |
| Q4 ≥ 2.79 | 2054 | 171 | 1.00 | 1.00 | 1.00 | 115 | 1.00 | |
| P for Trend | < 0.0001 | 0.0133 | 0.7022 | 0.0107 | ||||
| During Pregnancy | ||||||||
| Q1 < 2.29 | 1914 | 310 | 1.27 (1.19 - 1.35) | 1.02 (0.91 - 1.14) | 105 | 1.04 (0.85 - 1.27) | 205 | 1.02 (0.90 - 1.16) |
| Q2 2.29 - 3.03 | 2000 | 224 | 1.19 (1.07 - 1.32) | 1.01 (0.88 - 1.16) | 72 | 0.99 (0.76 - 1.29) | 152 | 1.02 (0.87 - 1.20) |
| Q3 3.03 - 3.88 | 2042 | 182 | 1.12 (0.90 - 1.40) | 0.94 (0.74 - 1.20) | 54 | 0.85 (0.53 - 1.37) | 128 | 0.99 (0.74 - 1.31) |
| Q4 ≥ 3.88 | 2061 | 164 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| P for Trend | < 0.0001 | 0.1183 | 0.7695 | 0.0999 | ||||
| First Trimester | ||||||||
| Q1 < 2.21 | 1920 | 305 | 1.22 (1.14 - 1.30) | 0.97 (0.88 - 1.07) | 107 | 0.92 (0.76 - 1.10) | 198 | 1.00 (0.89 - 1.13) |
| Q2 2.21 - 2.97 | 2002 | 222 | 1.12 (1.01 - 1.24) | 0.91 (0.79 - 1.04) | 73 | 0.91 (0.71 - 1.18) | 149 | 0.91 (0.78 - 1.06) |
| Q3 2.97 - 3.80 | 2052 | 172 | 0.95 (0.76 - 1.18) | 0.74 (0.58 - 0.94) | 45 | 0.56 (0.34 - 0.91) | 127 | 0.81 (0.62 - 1.07) |
| Q4 ≥ 3.80 | 2043 | 181 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| P for Trend | < 0.0001 | 0.6252 | 0.9533 | 0.6102 | ||||
| Second Trimester | ||||||||
| Q1 < 2.30 | 1923 | 301 | 1.23 (1.15 - 1.31) | 0.96 (0.86 - 1.06) | 104 | 0.98 (0.81 - 1.19) | 197 | 0.96 (0.85 - 1.09) |
| Q2 2.30 - 3.05 | 2008 | 217 | 1.13 (1.02 - 1.25) | 0.92 (0.80 - 1.05) | 71 | 0.99 (0.77 - 1.29) | 146 | 0.90 (0.77 - 1.05) |
| Q3 3.05 - 3.93 | 2036 | 188 | 1.09 (0.88 - 1.35) | 0.91 (0.72 - 1.16) | 53 | 0.83 (0.52 - 1.33) | 135 | 0.96 (0.73 - 1.26) |
| Q4 ≥ 3.93 | 2050 | 174 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| P for Trend | < 0.0001 | 0.7281 | 0.9885 | 0.6682 | ||||
| Third Trimester | ||||||||
| Q1 < 2.28 | 1902 | 322 | 1.29 (1.21 - 1.38) | 1.05 (0.94 - 1.17) | 107 | 1.07 (0.88 - 1.30) | 215 | 1.05 (0.92 - 1.19) |
| Q2 2.28 - 3.04 | 2014 | 211 | 1.15 (1.03 - 1.28) | 1.00 (0.87 - 1.14) | 72 | 1.05 (0.81 - 1.36) | 139 | 0.98 (0.83 - 1.15) |
| Q3 3.04 - 3.92 | 2040 | 184 | 1.14 (0.92 - 1.42) | 0.99 (0.78 - 1.26) | 51 | 0.78 (0.49 - 1.25) | 133 | 1.08 (0.82 - 1.43) |
| Q4 ≥ 3.92 | 2061 | 163 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| P for Trend | < 0.0001 | 0.0335 | 0.5677 | 0.0351 | ||||
a OR univariate analyses.
b OR adjusted for weight gain during pregnancy, total energy intake, monthly income per capita, maternal education level, smoking, maternal employment, multivitamin supplement, gestational hypertension, history of premature birth, reproductive history and dietary zinc intake.
4.3. Associations of Maternal Dietary Zinc Intake with the Risk of Preterm
As shown in Table 2, zinc intake was positively associated with the risk of preterm labor. The results of the trend test for dietary zinc intake during pregnancy were significant, with adjusted ORs of 1.29 (1.09 - 1.52), 1.55 (1.13 - 2.12), and 1.20 (1.00 - 1.46) for quartile 2 compared to quartile 4 throughout the pregnancy. There was a significant association between zinc intake and preterm labor, including spontaneous preterm labor. Additionally, quartile 2 had a significantly higher risk of preterm labor in the second and third trimesters of pregnancy compared to quartile 4. However, no significant association between zinc intake and medically induced preterm labor was observed, either before pregnancy or when stratified by trimester.
| Dietary Zinc Intake (mg/d) | Term (N = 8017) | Preterm (N = 880) | Medically Indicated | Spontaneous | ||||
|---|---|---|---|---|---|---|---|---|
| Cases | OR a (95% CI) | OR b (95% CI) | Cases | OR b (95% CI) | Cases | OR b (95% CI) | ||
| Before pregnancy | ||||||||
| Q1 < 5.21 | 1894 | 330 | 1.30 (1.22 - 1.39) | 1.09 (0.99 - 1.20) | 121 | 1.12 (0.94 - 1.34) | 209 | 1.06 (0.94 - 1.19) |
| Q2 5.21 - 6.73 | 2018 | 207 | 1.14 (1.03 - 1.27) | 1.02 (0.89 - 1.17) | 59 | 1.07 (0.83 - 1.38) | 148 | 1.01 (0.86 - 1.18) |
| Q3 6.73 - 8.47 | 2043 | 181 | 1.13 (0.90 - 1.41) | 1.13 (0.88 - 1.44) | 48 | 0.92 (0.57 - 1.47) | 133 | 1.21 (0.91 - 1.60) |
| Q4 ≥ 8.47 | 2062 | 162 | 1.00 | 1.00 | 50 | 1.00 | 112 | 1.00 |
| P for trend | < 0.0001 | 0.0038 | 0.1037 | 0.0312 | ||||
| During pregnancy | ||||||||
| Q1 < 6.53 | 1873 | 352 | 1.37 (1.28 - 1.46) | 1.09 (0.94 - 1.26) | 118 | 0.93 (0.71 - 1.23) | 234 | 1.14 (0.97 - 1.36) |
| Q2 6.53 - 8.27 | 2005 | 219 | 1.22 (1.10 - 1.36) | 1.29 (1.09 - 1.52) | 71 | 1.55 (1.13 - 2.12) | 148 | 1.20 (1.00 - 1.46) |
| Q3 8.27 - 10.28 | 2067 | 157 | 1.04 (0.82 - 1.31) | 0.97 (0.74 - 1.26) | 47 | 0.87 (0.52 - 1.48) | 110 | 1.01 (0.75 - 1.36) |
| Q4 ≥ 10.28 | 2072 | 152 | 1.00 | 1.00 | 42 | 1.00 | 110 | 1.00 |
| P for trend | < 0.0001 | < 0.0001 | 0.1471 | < 0.0001 | ||||
| First trimester | ||||||||
| Q1 < 6.30 | 1894 | 331 | 1.31 (1.23 - 1.40) | 1.01 (0.89 - 1.16) | 117 | 1.01 (0.79 - 1.30) | 214 | 1.01 (0.86 - 1.18) |
| Q2 6.30 - 8.03 | 1994 | 229 | 1.22 (1.10 - 1.35) | 1.13 (0.97 - 1.32) | 70 | 1.22 (0.90 - 1.64) | 159 | 1.10 (0.92 - 1.31) |
| Q3 8.03 - 10.01 | 2065 | 160 | 1.00 (0.80 - 1.26) | 0.90 (0.70 - 1.17) | 47 | 0.97 (0.58 - 1.62) | 113 | 0.90 (0.67 - 1.21) |
| Q4 ≥ 10.01 | 2064 | 160 | 1.00 | 1.00 | 44 | 1.00 | 116 | 1.00 |
| P for trend | < 0.0001 | 0.0227 | 0.2728 | 0.0537 | ||||
| Second trimester | ||||||||
| Q1 < 6.57 | 1888 | 336 | 1.36 (1.25 - 1.43) | 1.07 (0.92 - 1.23) | 118 | 0.95 (0.73 - 1.23) | 218 | 1.11 (0.94 - 1.32) |
| Q2 6.57 - 8.38 | 2000 | 224 | 1.22 (1.10 - 1.36) | 1.18 (1.00 - 1.38) | 75 | 1.39 (1.04 - 1.85) | 149 | 1.10 (0.91 - 1.33) |
| Q3 8.38 - 10.47 | 2059 | 165 | 1.07 (0.85 - 1.34) | 1.01 (0.78 - 1.30) | 40 | 0.74 (0.44 - 1.27) | 125 | 1.11 (0.83 - 1.49) |
| Q4 ≥ 10.47 | 2070 | 155 | 1.00 | 1.00 | 45 | 1.00 | 110 | 1.00 |
| P for trend | < 0.0001 | 0.0055 | 0.1351 | 0.0251 | ||||
| Third trimester | ||||||||
| Q1 < 6.53 | 1878 | 346 | 1.36 (1.27 - 1.46) | 1.10 (0.96 - 1.26) | 117 | 1.02 (0.80 - 1.31) | 229 | 1.13 (0.96 - 1.32) |
| Q2 6.53 - 8.36 | 2001 | 224 | 1.24 (1.11 - 1.38) | 1.27 (1.09 - 1.48) | 73 | 1.48 (1.10 - 1.97) | 151 | 1.19 (0.99 - 1.43) |
| Q3 8.36 - 10.48 | 2064 | 159 | 1.06 (0.84 - 1.33) | 1.06 (0.82 - 1.38) | 46 | 1.01 (0.60 - 1.67) | 113 | 1.09 (0.81 - 1.47) |
| Q4 ≥ 10.48 | 2074 | 151 | 1.00 | 1.00 | 42 | 1.00 | 109 | 1.00 |
| P for trend | < 0.0001 | < 0.0001 | 0.1000 | 0.0004 | ||||
a OR univariate analyses.
b OR adjusted for weight gain during pregnancy, total energy intake, monthly income per capita, maternal education level, smoking, maternal employment, multivitamin supplement, gestational hypertension, history of premature birth, reproductive history and dietary zinc intake.
4.4. Interaction Effects of Maternal Dietary Copper and Zinc Intake on the Risk of Preterm
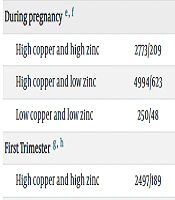
We further examined the interaction between maternal dietary intake of copper and zinc and the risk of preterm labor. After stratifying by trimester, we observed an additive effect between low dietary intake of copper and zinc in the second trimester and preterm labor. The maternal synergy Index was 4.95 (S = 4.95, 95% CI: 3.19 - 7.66), the relative excess hazard ratio attributable to the additive interaction was 2.51 (RERI = 2.51, 95% CI: 1.39 - 3.64), and 61% (AP = 0.61, 95% CI: 0.50 - 0.71) of the risk of low gestational body weight could be attributed to the interaction between low maternal dietary copper and zinc intake. There was also a multiplicative interaction between low dietary copper intake and low dietary zinc intake (OR = 2.23, 95% CI: 1.64 - 3.04) (Table 3).
| Maternal Dietary Intake | Term/Preterm | OR b (95% CI) | OR c (95% CI) | |
|---|---|---|---|---|
| Before Pregnancy d | ||||
| High copper and high zinc | 3004/245 | 1.00 | 1.00 | |
| High copper and low zinc | 4610/567 | 1.23 (1.14 - 1.33) | 1.07 (0.98 - 1.17) | |
| Low copper and low zinc | 401/68 | 2.08 (1.56 - 2.77) | 1.19 (0.81 - 1.76) | |
| During pregnancy e, f | ||||
| High copper and high zinc | 2773/209 | 1.00 | 1.00 | |
| High copper and low zinc | 4994/623 | 1.29 (1.19 - 1.40) | 1.07 (0.97 - 1.19) | |
| Low copper and low zinc | 250/48 | 2.55 (1.82 - 3.58) | 1.47 (0.73 - 3.00) | |
| First Trimester g, h | ||||
| High copper and high zinc | 2497/189 | 1.00 | 1.00 | |
| High copper and low zinc | 5247/637 | 1.27 (1.16 - 1.38) | 1.06 (0.96 - 1.18) | |
| Low copper and low zinc | 273/54 | 2.61 (1.89 - 3.63) | 1.41 (0.73 - 2.73) | |
| Second Trimester i, j | ||||
| High copper and high zinc | 2915/222 | 1.00 | 1.00 | |
| High copper and low zinc | 4848/609 | 1.28 (1.19 - 1.39) | 1.08 (0.98 - 1.19) | |
| Low copper and low zinc | 253/49 | 2.54 (1.82 - 3.56) | 1.40 (0.74 - 2.67) | |
| Third Trimester k, l | ||||
| High copper and high zinc | 2919/216 | 1.00 | 1.00 | |
| High copper and low zinc | 4847/587 | 1.28 (1.18 - 1.39) | 1.10 (1.00 - 1.22) | |
| Low copper and low zinc | 251/77 | 4.15 (3.10 - 5.54) | 3.70 (2.04 - 6.71) | |
a No case data were available for the low copper and high zinc groups.
b OR univariate analyses.
c OR adjusted for weight gain during pregnancy, total energy intake, monthly income per capita, maternal education level, smoking, maternal employment, multivitamin supplement, gestational hypertension, history of premature birth and reproductive history. Multiplicative Interaction: ORd (95% CI) = 1.16 (0.87 - 1.56), P = 0.31.
d Additive Interaction: RERI (95% CI) = 1.57 (1.01 - 2.13), AP (95% CI) = 0.76 (0.64 ~ 0.87), S (95% CI) = - 2.19.
e Multiplicative interaction: ORd (95% CI) =1.24 (0.87 - 1.78), P = 0.24.
f Additive Interaction: RERI (95% CI) = 0.89 (0.08 - 1.70), AP (95% CI) = 0.35 (0.14 - 0.56), S (95% CI) =2.36 (1.34 - 4.15).
g Multiplicative Interaction: ORd (95% CI) = 1.30 (0.92 - 1.84), P = 0.13.
h Additive Interaction: RERI (95% CI) = 1.01 (- 0.75 - 2.77), AP (95% CI) = 0.39 (- 0.03 - 0.80), S (95% CI) =2.67 (0.87 - 8.17).
i Multiplicative Interaction: ORd (95% CI) = 1.26 (0.88 - 1.79), P = 0.21.
j Additive Interaction: RERI (95% CI) = 1.89 (1.09 - 2.70), AP (95% CI) = 0.75 (0.63 - 0.86), S (95% CI) = - 4.40.
k Multiplicative Interaction: ORd (95% CI) = 2.23 (1.64 - 3.04), P < 0.001.
l Additive Interaction: RERI (95% CI) = 2.51 (1.39 - 3.64), AP (95% CI) = 0.61 (0.50 - 0.71), S (95% CI) = 4.95 (3.19 - 7.66).
5. Discussion
In this nested cohort study, we observed a significantly increased risk of preterm labor in women with low dietary copper intake before and during pregnancy, demonstrating a dose - response relationship, particularly for spontaneous preterm labor. Similarly, there was a significant inverse association between lower dietary zinc intake during pregnancy and preterm labor. Additionally, there were positive additive and multiplicative interactions between low dietary copper and zinc intakes and preterm labor during the preconception period and different trimesters of pregnancy. However, the low intake of copper and high intake of zinc were not statistically significant. To our knowledge, this study represents the first comprehensive investigation into the impact of dietary copper and zinc intake on preterm labor spanning from preconception to full gestation, offering valuable insights for future research in this area.
Few previous studies have investigated the effects of dietary copper intake on preterm birth, and most have focused on nutritional supplement interventions, yielding controversial and inconclusive results on whether dietary copper intake reduces the risk of preterm birth (16-21). Most studies report that low copper levels during pregnancy are associated with the risk of preterm birth. A case-control study in Gonabad, Iran, found that preterm mothers had significantly lower serum copper levels (149.82 ± 53.13 µg/dL) compared to full - term mothers (183.97 ± 71.40 µg/dL), suggesting that elevated maternal copper levels in early pregnancy may be linked to spontaneous preterm labor (16). In a prospective nested case - control study in China, the serum copper concentration in the preterm labor group (median: 184 µg/dL) was significantly higher than in the non - preterm labor group (median: 166 µg/dL, P < 0.0001), suggesting that elevated maternal copper levels in early pregnancy may elevate the risk of spontaneous preterm labor by increasing plasma TC and TG concentrations (17). However, two other studies found no association between maternal copper levels during pregnancy and the risk of developing preterm labor (20, 21). Meanwhile, there are conflicting findings regarding the relationship between dietary zinc intake and the risk of preterm labor (13, 22-24). A meta-analysis of 16 randomized controlled trials found that zinc supplementation resulted in a 14% reduction in preterm birth (RR = 0.86, CI = 0.76 - 0.97) (22). However, some studies show that zinc supplementation during pregnancy is not associated with a reduced risk of preterm birth (23, 24). Based on 25 randomized controlled trials involving over 18,000 women and their babies, half with a low overall risk of bias, evidence suggests that zinc supplementation may have minimal impact on reducing the risk of preterm labor (RR: 0.87, 95% CI: 0.74 - 1.03) (13). Our findings suggest that lower intake of both copper and zinc before and during pregnancy may have an additive effect on the risk of preterm labor. Given the paucity of studies on the joint effect of copper and zinc intake on the risk of developing preterm labor, further research is necessary to confirm and interpret these findings. According to studies, it has been found that preterm labor is associated with oxidative stress, and therefore preterm women have higher levels of oxidative stress in their bodies (26). Oxidative stress is caused by an imbalance between free radicals and antioxidants (27). Existing research suggests that oxidative stress reduces cellular defenses and destroys collagen in the embryonic membranes, leading to preterm birth (28). Trace elements such as zinc and copper are involved in the synthesis of DNA, RNA, and collagen, and play a key role in reducing peroxidation and oxidative processes. Zinc functions as an antioxidant by stabilizing membrane structure, safeguarding sulfhydryl groups in proteins, upregulating the expression of metallothionein, and inhibiting anti-inflammatory responses (29). Copper is a reactive oxidizing metal that reduces oxidation by catalyzing hydroxyl radicals (30). Moreover, inadequate copper and zinc levels may impair placental function, hindering nutrient transfer and oxygen exchange between the mother and fetus (31). The antioxidant effects of zinc and copper may be a potential mechanism for reducing the incidence of preterm labor. Furthermore, potential vulnerability factors, such as maternal health conditions, socio - economic status, access to healthcare, dietary habits, and genetic predispositions, can also influence nutrient absorption, utilization, and overall maternal and fetal health (32). Another important factor to consider is that the bioavailability and metabolic fates of copper and zinc may differ between dietary and supplemental sources. Factors such as the presence of dietary fibers, phytates, and other minerals may enhance or inhibit the absorption of copper and zinc from food sources (33, 34). In contrast, supplements typically provide isolated forms of copper and zinc, often in highly bioavailable forms such as copper gluconate or zinc sulfate (35). These supplements bypass the potential inhibitory factors present in food and are absorbed more efficiently in the digestive tract. In the future, dietary counseling, fortification, or supplementation strategies may be necessary to optimize nutritional status and reduce the risk of copper and zinc deficiency in pregnant women, ultimately aiming to prevent premature birth. Our study has several limitations. Firstly, we lack actual biomarker data for copper and zinc status in study participants, relying solely on self - reported dietary intake information, which inevitably introduces recall bias and may not fully capture the absorbed and utilized amounts of these minerals. Future research is encouraged to include biomarker measurements to enhance the robustness of the findings. Secondly, the interaction between dietary multivitamins and trace elements cannot be quantitatively analyzed, which may lead to confounding effects. To address this, strict quality control measures were implemented throughout the entire process, from questionnaire design to data entry, to minimize recall bias. Additionally, detailed demographic, medical history, and lifestyle information allowed us to adjust for and control confounders. The diagnosis of preterm birth and its subtypes was based on medical records rather than self - reports, minimizing the potential risk of misdiagnosis. Another limitation is the challenge of discerning the specific effects of copper and zinc from dietary sources versus supplementation. Finally, adjusting for the intake of macronutrients in addition to energy intake could provide valuable insights; however, we regret that we do not possess relevant data to perform such adjustments in our study.
5.1. Conclusions
Our study suggests that a lower intake of copper and zinc in the diet before and during pregnancy may increase the risk of preterm birth. Additionally, a low intake of these minerals during pregnancy appears to have a synergistic effect on this risk. These findings highlight the importance of promoting adequate copper and zinc intake before and during pregnancy to reduce the incidence of preterm births in Lanzhou City. To confirm these findings and elucidate the underlying mechanisms, future human studies should include data on maternal biomarkers, mineral intake, and genetics.