1. Background
Pediatric shock is a leading cause of death in children worldwide, with over 10 million deaths reported annually. Shock occurs when body tissues do not receive adequate oxygen and nutrients, resulting in tissue hypoxia (1). It can be classified into four types: Hypovolemic, cardiogenic, distributive, and obstructive shock. Previous studies have shown that septic shock and cardiogenic shock are the most common in pediatric patients (2, 3).
In April 2020, reports from the United Kingdom documented a presentation in children similar to incomplete Kawasaki disease (KD) or toxic shock syndrome. This condition has been termed multisystem inflammatory syndrome in children associated with COVID-19 (MIS-C) (4-8). A recent study demonstrated that the mean age was 9 years, and fever, rash, and gastrointestinal symptoms were common presentations in MIS-C patients (9). In the multicenter study, there was a higher proportion of hospitalizations with shock in MIS-C compared with KD (10). A systematic review found that up to 79.3% of patients had cardiac and vascular system impacts. Shock occurred in 59.9% of patients, with 73.3% requiring treatment in intensive care units. The reported mortality rate was 1.9% (7, 8, 11). Several studies have reported that patients diagnosed with MIS-C who experience shock may present with symptoms consistent with both vasodilatory and cardiogenic shock, with a higher incidence of vasodilatory shock (11, 12). The diagnosis of MIS-C shock is challenging due to its similarity to septic shock and cardiogenic shock. However, there is a lack of comparative studies investigating the differences between MIS-C with shock, septic shock, and cardiogenic shock.
2. Objectives
The aim of our study was to investigate the clinical characteristics of patients with MIS-C shock and compare them with patients with septic shock and cardiogenic shock. Additionally, we aimed to evaluate the treatment approaches and outcomes associated with these three types of shock.
3. Methods
This retrospective study was conducted in the pediatric intensive care unit (PICU) at Queen Sirikit National Institute of Child Health (QSNICH) in Bangkok from January 2021 to December 2022. The aim of the study was to analyze patients aged 1 month to 15 years admitted with MIS-C shock, septic shock, or cardiogenic shock. The diagnostic criteria for MIS-C were based on the Centers for Disease Control and Prevention (CDC) definition (13): (1) fever ≥ 38°C or subjective fever for ≥ 24 hours, (2) laboratory evidence of inflammation (C-reactive protein, erythrocyte sedimentation rate, fibrinogen, D-dimer, ferritin, LDH, interleukin-6, neutrophilia, and hypoalbuminemia), (3) severe illness requiring hospitalization, (4) involvement of ≥ 2 organ systems (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, and neurologic), (5) no other plausible diagnosis, and (6) SARS-CoV-2 infection or exposure (positive PCR serology or antigen test, or COVID-19 exposure within 4 weeks before onset). Sepsis was defined as severe organ dysfunction resulting from an unregulated host response to infection, and septic shock was a subset of sepsis characterized by cardiovascular dysfunction, including hypotension, use of vasoactive drugs, or impaired perfusion (14). The diagnosis of cardiogenic shock was defined as: (1) clinical signs (tachycardia, dyspnea, jugular vein distention, and hepatomegaly), and (2) evidence of myocardial dysfunction by echocardiography (15, 16). Both MIS-C shock and cardiogenic shock groups underwent echocardiography performed by cardiologists. Patients with cardiac arrest and insufficient data in the medical records were excluded from the study. Medical records were reviewed to capture demographic information, clinical characteristics, laboratory and imaging features, treatment approaches, and outcomes. Ethical approval for the study had been obtained from the Institute's ethics committees.
Statistical analysis was performed using IBM SPSS statistics version 26.0. Median (interquartile range) was used for continuous variables, and percentages were used for categorical variables. For the comparison of groups of categorical variables, either the chi-square test or Fisher's exact test was used. For non-normally distributed continuous data, the Kruskal-Wallis test or Mann-Whitney U test was used, and for normally distributed continuous data, ANOVA was used. A significance level of P < 0.05 was considered statistically significant.
4. Results
A total of 60 patients were enrolled in this study, and their baseline and clinical characteristics are shown in Table 1. Among all participants, 21.6% were classified in the MIS-C shock group. Patients in the cardiogenic shock group had significantly lower body temperatures than the other groups (P < 0.001). Skin manifestations occurred more frequently in patients with MIS-C shock (P < 0.001). There was a higher percentage of patients with hematologic system involvement in the septic shock group (P = 0.016). Regarding underlying comorbidities, nearly all patients in the MIS-C shock group had none (P = 0.003). No significant differences were found between the three groups in terms of gender, age, and Body Mass Index (BMI) (Table 1).
| Variables | MIS-C Shock N = 13 | Septic Shock N = 33 | Cardiogenic Shock N = 14 | P-Value |
|---|---|---|---|---|
| Age (y), median [IQR] | 8 [4 – 10] | 3 [2 – 11] | 1 [0.5 – 7] | 0.066 |
| Male | 9 (69.2) | 19 (57.6) | 8 (57.1) | 0.745 |
| Underlying | 0.003 b | |||
| Hematologic | 0 (0) | 10 (30.3) | 0 (0) | |
| Neurologic | 0 (0) | 11 (33.3) | 2 (14.3) | |
| Cardiovascular | 0 (0) | 2 (6.1) | 3 (21.4) | |
| Others | 1 (7.7) | 8 (24.2) | 1 (7.1) | |
| BMI, median [IQR] | 17.2 [13.4 - 23.1] | 16.3 [14.2 – 20] | 14 [13.1 – 19] | 0.449 |
| Body temperature (ºC) | 39 (38.8 - 39.9) | 39.4 (38.8 - 39.7) | 37 (36.9 - 38) | < 0.001 b |
| Clinical presentation | ||||
| Skin | 11 (84.6) | 6 (18.2) | 1 (7.1) | < 0.001 b |
| Cardiovascular | 12 (92.3) | 26 (78.8) | 14 (100) | 0.122 |
| Hematological c | 0 (0) | 11 (33.3) | 1 (7.1) | 0.016 |
| Gastrointestinal | 11 (84.6) | 21 (63.6) | 7 (50) | 0.169 |
| Respiratory | 8 (61.5) | 24 (72.7) | 13 (92.9) | 0.160 |
| Neurological d | 2 (15.4) | 9 (27.3) | 6 (42.9) | 0.286 |
| Nephrological | 2 (15.4) | 7 (21.2) | 6 (42.9) | 0.195 |
Abbreviations: BMI, Body Mass Index; MIS-C, multisystem inflammatory syndrome in children.
a Values are expressed as No (%).
b Statistically significant at P-value < 0.05 determined by Kruskal-Wallis test.
c Hematological presentation is petechiae.
d Neurological presentation is seizure.
4.1. Laboratory
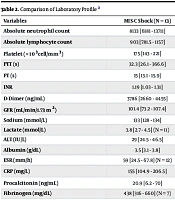
Laboratory analysis of hematologic parameters showed significant differences in absolute lymphocyte counts among the three groups, with a statistical significance of P = 0.001. The MIS-C shock group had a lower median absolute lymphocyte count (ALC) of 903 cells/μL compared with the septic shock group and the cardiogenic shock group. Patients in the cardiogenic shock group had significantly higher values for prothrombin time (PT) (P = 0.001) and international normalized ratio (INR) (P = 0.005).
As shown in Table 2, no significant differences were found between the three groups in blood urea nitrogen (BUN), creatinine (Cr), electrolyte levels, alanine transaminase (ALT), and lactate levels. However, C-reactive protein (CRP) (P = 0.001) and procalcitonin (PCT) (P = 0.012) levels were significantly higher in the MIS-C shock group. The septic shock group had higher LDH levels compared to the other groups (P = 0.002). Additionally, patients with cardiogenic shock had elevated troponin T levels (P = 0.002), as indicated in Table 2.
| Variables | MIS-C Shock (N = 13) | Septic Shock (N = 33) | Cardiogenic Shock (N = 14) | P-Value |
|---|---|---|---|---|
| Absolute neutrophil count | 8133 [6181 - 13711] | 6768 [780.5 - 13775] | 6148 [4222 - 10215] | 0.449 |
| Absolute lymphocyte count | 903 [781.5 - 1157] | 2156 [1004.5 - 2716.5] | 4990 [2107 - 6069] | 0.001 b |
| Platelet (× 103cell/mm3) | 175 [143 - 221] | 205 [47 - 350] | 220 [149 - 358] | 0.788 |
| PTT (s) | 32.3 [26.1 - 366.6] | 38.5 [32.8 - 43.5] | 37.8 [32.2 - 42.5] | 0.064 |
| PT (s) | 15 [13.1 - 15.9] | 16.3 [15 - 21.2] | 23 [16.8 - 28] | 0.001 b |
| INR | 1.19 [1.03 - 1.31] | 1.31 [1.19 - 1.74] | 1.71 [1.32 - 2.21] | 0.005 b |
| D-Dimer (ng/mL) | 3786 [2660 - 4455] | 3948 [1008 - 5500] | 5210 [1493 - 12077] | 0.536 |
| GFR (mL/min/1.73 m2) | 101.4 [73.2 - 107.4] | 92.7 [70.8 - 142.4] | 70.1 [42.6 - 94.8] | 0.065 |
| Sodium (mmol/L) | 133 [128 - 134] | 135 [130.5 - 140] | 137 [127.7 - 139] | 0.078 |
| Lactate (mmol/L) | 3.8 [2.7 - 4.5] (N = 11) | 3.6 [2.5 - 4.8] (N = 28) | 3.1 [1.6 - 5.4] | 0.779 |
| ALT (IU/L) | 29 [24.5 - 46.5] | 34 [13.5 - 132] | 25.5 [14.7 - 125.5] | 0.829 |
| Albumin (g/dL) | 3.5 [3.1 - 3.8] | 3.3 [2.7 - 3.8] | 3.4 [3.0 - 3.9] | 0.837 |
| ESR (mm/h) | 59 [24.5 - 67.8] (N = 12) | 23.5 [13.2 - 86.3] (N = 4) | 1 [1 - 60.5] (N = 5) | 0.122 |
| CRP (mg/L) | 155 [104.9 - 206.5] | 152 [43.5 - 269] (N = 8) | 10.2 [0.2 - 72] (N = 6) | 0.010 b |
| Procalcitonin (ng/mL) | 20.9 [6.2 - 70] | 4.9 [0.6 - 64] (N = 25) | 0.9 [0.2 - 3.9] (N = 9) | 0.012 b |
| Fibrinogen (mg/dL) | 438 [316 - 660] (N = 7) | 371 [163 - 674] (N = 7) | 128 [124 - 291] (N = 5) | 0.059 |
| Ferritin (× 103ng/mL) | 1.3 [0.8 - 2.1] (N = 12) | 7.6 [0.4 - 31.7] (N = 5) | 0.5 [0.04 - 1.7] (N = 3) | 0.304 |
| LDH(U/L) | 368 [306 - 447] (N = 11) | 563.5 [497 - 1585] (N = 8) | 217.5 [103 - 217.5] (N = 2) | 0.002 b |
| Pro-BNP (× 103pg/mL) | 9.2 [5.4 - 19] (N = 11) | 0.6 [0.3 - 26.7] (N = 4) | 35 [3.8 - 84.6] (N = 3) | 0.193 |
| Troponin T (ng/L) | 41.5 [25.3 - 94.5] (N = 12) | 12.4 [4.5 - 27.5] (N = 5) | 211.5 [61.3 - 403] (N = 8) | 0.002 b |
Abbreviation: PTT, partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; GFR, glomerular filtration rate; ALT, alanine transaminase; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; LDH, lactate dehydrogenase; Pro-BNP, N-terminal pro B-type natriuretic peptide.
a Values are expressed as median [IQR].
b Statistically significant at P-value < 0.05 determined by Kruskal-Wallis test.
4.2. Imaging and Hemodynamic Findings
Abnormal chest radiographs were more common in MIS-C shock patients (84.6%) and cardiogenic shock patients (100%) than in septic shock patients (48.5%), with a statistically significant difference of P = 0.004. The most common abnormality observed in the MIS-C shock and cardiogenic shock groups was cardiomegaly with increased pulmonary blood flow. Significant differences in echocardiogram results were observed between the groups (P = 0.001). In the MIS-C shock group, 30.8% of patients had coronary abnormalities, and 61.5% showed poor cardiac function. All patients (100%) in the cardiogenic shock group had poor cardiac function. Ultrasonic cardiac output monitor (USCOM) results also showed a significant difference (P = 0.004) between the groups. In the MIS-C shock group, 63.6% of patients had a low Inotropy Index (iNO) and systemic vascular resistance index (SVRI). In the cardiogenic shock group, 66.6% had low iNO, whereas in the septic shock group, 53.3% had low SVRI (Table 3).
| Variables | MIS-C Shock | Septic Shock | Cardiogenic Shock | P- Value |
|---|---|---|---|---|
| Chest radiographic findings | N = 13 | N = 33 | N = 14 | 0.004 b |
| Normal | 2 (15.4) | 17 (51.5) | 0 (0) | |
| Cardiomegaly with increased PBF | 11 (84.6) | 0 (0) | 11 (78.6) | |
| Cardiomegaly with decreased PBF | 0 (0) | 0 (0) | 3 (21.4) | |
| Lobar opacity | 0 (0) | 9 (27.3) | 0 (0) | |
| Multilobar opacity | 0 (0) | 1 (3) | 0 (0) | |
| Perihilar opacity | 0 (0) | 3 (9.1) | 0 (0) | |
| Bilateral ground glass | 0 (0) | 3 (9.1) | 0 (0) | |
| Echocardiographic results | N = 13 | N = 30 | N = 14 | 0.001 b |
| Normal | 1 (7.7) | 24 (80) | 0 (0) | |
| Poor LVEF | 8 (61.5) | 6 (20) | 13 (92.9) | |
| Coronary abnormalities | 4 (30.8) | 0 (0) | 1 (7.1) | |
| USCOM findings | N = 11 | N = 30 | N = 6 | 0.004 b |
| Normal | 2 (18.2) | 7 (23.3) | 1 (16.7) | |
| Low iNO | 2 (18.2) | 3 (10) | 4 (66.6) | |
| Low SVRI | 0 (0) | 16 (53.3) | 0 | |
| Low iNO and SVRI | 7 (63.6) | 4 (13.4) | 1 (16.7) |
Abbreviation: PBF, pulmonary blood flow; LVEF, left ventricular ejection fraction; USCOM, ultrasound cardiac output monitoring; iNO, Inotropy Index (W/m2); SVRI, systemic Vascular Resistant Index (ds cm-5 m2).
a Values are expressed as No (%).
b Statistically significant at P-value < 0.05 determined by Kruskal-Wallis test.
4.3. Treatment
Table 4 shows a significant difference in initial fluid resuscitation between the different patient groups (P < 0.001). In the MIS-C shock group, 53.8% of patients received fluid volumes between 10 and 20 mL/kg. In the septic shock group, 42.4% of patients received fluid volumes between 20 and 40 mL/kg. In contrast, in the cardiogenic shock group, 35.1% of patients did not receive fluid resuscitation.
Notable differences were found in the initial use of vasoactive drugs between groups (P < 0.001). The use of dobutamine was higher in the MIS-C shock and cardiogenic shock groups, with rates of 76.9% and 57.2%, respectively. In contrast, the septic shock group predominantly received norepinephrine as the primary medication, with a use rate of 65.4% (Table 4).
A statistically significant difference (P = 0.002) was found in the use of ventilatory support among the three groups. In the cardiogenic shock group, 100% used mechanical ventilators, compared to 69.7% in the septic shock group. Conversely, the MIS-C shock group had the lowest rate of invasive mechanical ventilation, with only 30.8% (Table 4).
| Variables | MIS-C Shock | Septic Shock | Cardiogenic Shock | P-Value |
|---|---|---|---|---|
| Fluid resuscitation | N = 13 | N = 33 | N = 14 | < 0.001 b |
| No (mL/kg) | 2 (15.4) | 0 (0) | 5 (35.1) | |
| 10 - 20 | 7 (53.8) | 7 (21.2) | 4 (28.6) | |
| > 20 - 40 | 3 (23.1) | 14 (42.4) | 4 (28.6) | |
| > 40 - 60 | 0 (0) | 7 (21.2) | 1 (7.1) | |
| > 60 | 1 (7.7) | 5 (15.2) | 0 (0) | |
| Initial vasoactive drugs | N = 13 | N = 26 | N = 14 | < 0.001 b |
| Norepinephrine | 0 (0) | 17 (65.4) | 0 (0) | |
| Epinephrine | 3 (23.1) | 7 (26.9) | 3 (21.4) | |
| Dobutamine | 10 (76.9) | 2 (7.7) | 8 (57.2) | |
| Dopamine | 0 (0) | 0 (0) | 3 (21.4) | |
| Respiratory support | N = 13 | N = 33 | N = 14 | 0.003 b |
| Cannula | 4 (30.8) | 8 (24.2) | 0 (0) | |
| HFNC/NIV | 5 (38.5) | 2 (6.1) | 0 (0) | |
| Invasive mechanical ventilator | 4 (30.8) | 23 (69.7) | 14 (100) | |
| Outcome | N = 13 | N = 33 | N = 14 | |
| Death | 0 (0) | 6 (18.2) | 5 (35.7) | 0.059 |
| Length of stay (days), median [IQR] | 7 [7 - 13] | 22 [12 - 38] | 25 [5 - 37] | 0.016 b |
| Length of PICU stay (days), median [IQR] | 3 [3 - 5] | 5 [3 - 12] | 9 [4 - 23] | 0.028 b |
| Complications | N= 13 | N = 33 | N = 14 | 0.150 |
| Hospital acquired infection | 0 (0) | 6 (18.2) | 4 (28.6) | |
| Cardiovascular | 6 (46.2) | 0 (0) | 1 (7.1) | |
| Others | 1 (7.7) | 7 (21.2) | 5 (35.7) |
Abbreviation: HFNC, high flow nasal cannula; NIV, non-invasive ventilation.
a Values are expressed as No (%).
b statistically significant at P-value < 0.05 determined by Kruskal-Wallis test
All patients with MIS-C shock received treatment with glucocorticoids, intravenous immunoglobulin (IVIG), and antiplatelet agents.
4.4. Outcome
No deaths were reported in the MIS-C shock group. Additionally, this group had a shorter length of stay in the PICU (P = 0.028) and an overall shorter hospital stay (P = 0.016) compared to the other two groups. Remarkably, there were no significant differences in complications, including hospital-acquired infections and cardiovascular sequelae, among the three groups.
5. Discussion
This study represents a novel contribution by performing a comparative analysis of MIS-C shock, septic shock, and cardiogenic shock. Our findings regarding the demographic characteristics, including age, sex, and comorbidities, of patients with MIS-C shock are consistent with previous reports (17-20).
We found that MIS-C shock was characterized by specific demographic features and clinical presentations, such as an age range of 4 to 10 years and the presence of mucocutaneous involvement. A similar previous study reported that the median age of patients was 7 years and that fever, rash, and abdominal pain were the most common clinical presentations (21). In the MIS-C shock group, all children had higher CRP and PCT levels and lower lymphocyte counts, consistent with previous research findings (17-19). These findings are particularly valuable for healthcare providers with limited diagnostic resources, as a targeted complete blood count (CBC) assessment to evaluate lymphocyte count and CRP can provide critical information. Notably, these markers show different patterns when compared with septic shock and cardiogenic shock, highlighting their importance in distinguishing these conditions. The pathophysiology of MIS-C shock is thought to be attributable to a hyperimmune response. Meanwhile, cardiogenic shock and septic shock involve hemodynamic and circulatory disturbances, triggering systemic inflammation and organ dysfunction. Consequently, inflammatory markers may elevate in these groups. However, in this study, the number of cardiogenic shock and septic shock cases was lower than those of MIS-C shock, which might affect the interpretation of inflammatory parameters. Therefore, increasing the sample size would enhance the validity of these findings.
Regarding lymphocyte count, we found that the MIS-C shock group exhibited significant lymphopenia compared to the other groups. This result aligns with previous studies and demonstrates that a low lymphocyte count is significantly associated with contractility dysfunction in MIS-C (22).
The results of our study suggest that patients with MIS-C shock and cardiogenic shock exhibit more frequent abnormalities on chest radiographs and echocardiography compared to patients with septic shock. These findings are consistent with previous studies by Einat Blumfield et al., who reported that cardiomegaly and congestive heart failure (63%) or cardiogenic pulmonary edema (56%) were the most common findings on chest radiographs in MIS-C cases (23). According to this study, poor left ventricular dysfunction was the most common cardiac finding. Similarly, recent studies showed that left ventricular systolic and diastolic dysfunction were present in MIS-C patients during the acute phase of the disease (24-26).
Our study also found that 30.8% of MIS-C shock patients had coronary abnormalities and 61.5% had poor cardiac function on echocardiography. These results are consistent with the studies by Caro-Domínguez et al., in which echocardiography revealed impaired cardiac function in 51% of cases and coronary artery anomalies in 14% of cases (27).
The USCOM device is a noninvasive instrument for measuring cardiac output using continuous Doppler ultrasound. Previous studies have shown a strong correlation between cardiac output measurements with the USCOM device and the thermodilution method with a pulmonary artery catheter (26). At QSNICH, the USCOM device is routinely used to assess the hemodynamic status of all patients with shock. However, there are currently no studies on the use of the USCOM device specifically in the MIS-C population. Therefore, our study is the first investigation of USCOM outcomes in patients with MIS-C shock. The results suggest that MIS-C shock patients exhibit a combination of myocardial dysfunction and vasodilatory shock based on USCOM results. According to a case series report, MIS-C shock appears to be a combination of distributive and cardiac dysfunction. An exaggerated immune response and hyperinflammatory state possibly affect the endothelium of vessels and the myocardium, leading to vasodilatory and ventricular dysfunction (11).
Early recognition, timely intervention, and close monitoring can improve the outcomes of shock. Hemodynamic monitoring and the assessment of fluid responsiveness using dynamic parameters are important in the management of shock (14, 28). Fluid therapy is crucial in managing critically ill patients with shock. The main goal of fluid resuscitation in shock is to improve cardiac output and organ perfusion, thereby reducing the risk of organ dysfunction. The objective is to optimize preload until the optimal forward stroke volume (SV) is achieved (29).
In our study, we found that the initial volume of fluid resuscitation was lower in patients with MIS-C shock and cardiogenic shock compared to patients with septic shock. This observation aligns with the underlying pathophysiology, as excessive fluid resuscitation in patients with cardiac impairment may exacerbate their condition, leading to increased morbidity and mortality. However, it should be noted that there are currently no established guidelines for fluid resuscitation specifically for the MIS-C group. As a result, fluid overload and the subsequent development of cardiogenic pulmonary edema are more common in many patients.
In this study, dobutamine was the primary vasoactive drug used as first-line treatment in patients with MIS-C shock and cardiogenic shock, whereas 65.4% of patients with septic shock received norepinephrine. The choice of drug was primarily based on physical examination findings and echocardiography results. In view of the USCOM findings and previous studies (11, 12, 30), it is evident that MIS-C shock involves a vasodilator component. The use of dobutamine, which has vasodilator effects, could potentially exacerbate the condition. However, we did not specifically investigate this aspect in our study.
Intravenous immunoglobulin and glucocorticoids are the most commonly used immunomodulatory medications in MIS-C. The American College of Rheumatology recommends using IVIG in combination with glucocorticoids as first-tier therapy for hospitalized children with MIS-C (31). The Thai guidelines recommend treating MIS-C shock with intravenous immune globulin 2 g/kg and pulse methylprednisolone. Additionally, low-dose aspirin should be used in patients with MIS-C and continued until the platelet count is normalized and normal coronary arteries are confirmed at > 4 weeks after diagnosis. In this study, we observed that all MIS-C shock patients received glucocorticoids, IVIG, and antiplatelet therapy. Notably, there were no reported fatalities in the MIS-C shock group. In contrast, the septic shock group had a mortality rate of 18.2%, and the cardiogenic shock group had a mortality rate of 35.7%. Furthermore, the MIS-C shock group had a significantly shorter hospital stay compared to the other two groups.
These findings suggest that our study achieved better outcomes compared to previous research (4, 11, 12). This improvement in outcomes may be attributed to the current availability of treatment guidelines and increased physician awareness, which facilitate the prompt administration of specific treatments. Interestingly, a previous study showed using a different dose of IVIG and steroids in MIS-C patients: 63.8% received IVIG at a dose of 1 g/kg, and 36.2% received at a dose of 2 g/kg. The results indicated good outcomes, as all patients had a proper response, no cardiac complications, and a low mortality rate. Therefore, they concluded that IVIG at the dose of 1 g/kg might be sufficient for treatment (32). Future research should further investigate the optimal dose of immunomodulatory medications in MIS-C and MIS-C shock. However, it is important to acknowledge several limitations of our study. Firstly, the retrospective nature of the study relied on data collected from medical records, which may contain inconsistencies and missing information. Additionally, incomplete investigations for some patients due to technical issues or the absence of indications may have impacted the comparisons between groups. Secondly, the sample size was relatively small, limiting the statistical power of our findings. Thirdly, the study was conducted at a single center, which may restrict the generalizability of the results. The patient population at our center may not represent the entire country, as our center is a tertiary care center, and many cases may have already received prior treatment. Fourthly, there is a risk of selection bias in our study because we selected patients from medical records. Finally, due to inter-observer variability in reporting the results of echocardiographic and USCOM findings, we could not conduct a statistical analysis. To overcome these limitations, future studies involving multiple centers can provide a more comprehensive understanding of these conditions in a broader population.
5.1. Conclusions
It is essential for physicians to consider MIS-C shock as a potential cause of shock in patients and distinguish it from septic shock and cardiogenic shock through additional investigations. Multisystem inflammatory syndrome in children shock patients present with skin manifestations, low ALC, elevated CRP and PCT levels, and hemodynamic evidence of vasodilatory shock with myocardial dysfunction. In healthcare facilities with limited diagnostic capabilities, basic laboratory tests such as CBC and CRP can aid in the diagnosis. In the absence of access to a cardiologist, if there is suspicion of MIS-C shock, adrenaline may be used as the initial vasoactive drug, given the evidence of both vasodilatory shock and myocardial dysfunction in MIS-C shock cases.