1. Background
Non-alcoholic fatty liver disease (NAFLD) is characterized by the absence of underlying factors that contribute to fat accumulation in the liver, such as alcohol consumption, prolonged use of medications that promote fat buildup, or monogenic hereditary disorders, and the presence of hepatic steatosis, as confirmed by imaging or histology (1). Hepatosteatosis is predicted to be the primary cause of late-term liver disease impacting children globally as obesity prevalence rises (2). A meta-analysis of research on the general population revealed that the prevalence of NAFLD was 7.6%, whereas studies on populations with obesity showed a much higher prevalence of 34.2% (3). Hepatosteatosis, which is correlated with metabolic syndrome, type 2 diabetes, and obesity, poses a risk for the development of chronic renal disease and cardiovascular disease in older age (2). Liver biopsy and imaging methods are used to diagnose NAFLD (4). In the presence of hepatosteatosis exceeding 30% of liver tissue, ultrasonographic findings become more pronounced. Despite its limitations, ultrasonography remains a valuable noninvasive tool for detecting hepatosteatosis and excluding alternative underlying etiologies (5). The diagnosis of NAFLD is now established by a process of exclusion due to the lack of clinically useful biomarkers available for diagnostic purposes (1).
Chronic inflammation is a major contributor to the development of NAFLD. According to the Multiple Impact Hypothesis, several factors, including dietary factors, gut microbiota, hormones produced by adipose tissue, insulin resistance, and genetic and epigenetic factors, together contribute to the development of NAFLD by causing oxidative stress and inflammation in the liver (6). Large amounts of free fatty acids reach the liver, causing mitochondrial dysfunction and increased production of reactive oxygen species (ROS). Reactive oxygen species can also lead to inflammation and fibrosis by activating Kupffer and hepatic stellate cells through lipid peroxidation (6). Therefore, inflammatory cytokines, neutrophil/lymphocyte ratio (NLR), thrombocyte/lymphocyte ratio (PLR), and lymphocyte/monocyte ratio (LMR) are used as potential biomarkers of inflammation for NAFLD (7, 8). Monocytes are inflammatory cells responsible for the increase in pro-inflammatory cytokines. Macrophage monocyte derivatives are key players in the pathophysiology of NAFLD and increase hepatic inflammatory responses (9). High-density lipoprotein cholesterol (HDL-C) exerts anti-inflammatory effects by inhibiting the pro-inflammatory and pro-oxidant actions of monocytes through blocking macrophage migration and LDL oxidation. HDL-C has also been shown to be effective in suppressing monocyte activation and preventing differentiation and proliferation. Studies have shown that the monocyte/HDL-C ratio (MHR), a newly developed measure of inflammation and oxidative stress, can be used as a reliable indication for predicting and assessing the prognosis of various diseases (10).
The definition of non-alcoholic fatty liver disease (NAFLD) has several restrictions. The terminology has been revised to metabolic (dysfunction)-associated fatty liver disease (MAFLD) to better align with adult NAFLD linked to metabolic abnormalities. MAFLD is characterized by the existence of cardiovascular and metabolic risk factors (CMRF) without any other underlying cause of hepatosteatosis (11, 12).
Recent studies in adults have used the monocyte-to-high-density lipoprotein cholesterol ratio (MHR) as a biomarker in the prediction of NAFLD (8, 13-15). To date, there has been only one study investigating MHR levels in children and adolescents with obesity. Haspolat and Unal demonstrated a significant elevation in MHR among children with obesity compared to their counterparts without obesity (16).
2. Objectives
The goal of this research is to investigate the correlation between MHR and MAFLD in children and adolescents with obesity and to assess the feasibility of using MHR as a novel and convenient biomarker for the detection of MAFLD.
3. Methods
The retrospective research, conducted using simple random sampling, included a cohort of individuals between the ages of 6 and 18, including children and adolescents, followed for obesity with a BMI > 95th percentile at the pediatric endocrinology clinic from 1 October 2022, to 30 September 2023. Demographic, anthropometric, laboratory, and ultrasound data were recorded from the patient files. Those with syndromic obesity and known liver disease-causing steatosis were not included in the study. Simple obesity in children and adolescents was defined as the absence of hepatosteatosis on ultrasonography and normal liver function. The diagnosis of pediatric MAFLD was defined when hepatosteatosis, confirmed by liver histology or imaging, was present in the absence of any other identifiable cause, along with the presence of any CMRF (11, 12).
Physical and pubertal examinations were performed in all cases. Weight measurements were carried out with a calibrated 100-gram-sensitive scale, and height measurements were made using a calibrated 1 millimeter-sensitive stadiometer (SECA, model 220, Hamburg, Germany). The Body Mass Index (kg/m²) was computed by dividing the weight by the square of the height. According to the reference curves prepared for Turkish children and adolescents, a BMI > 95th percentile was considered obese (17). The research received approval from the Ethics Board for Clinical Research at Ordu University (Ethics Board No. 2023/241).
Blood cell analyses were conducted using the XN 9000 analyzer (Sysmex Corporation, Kobe, Japan). The NLR was determined by dividing the count of neutrophils by the count of lymphocytes, the PLR by the count of thrombocytes, and the LMR by the count of lymphocytes. The calculation of the monocyte-to-HDL-C ratio involved dividing the absolute monocyte count (expressed in 109/L) by the HDL-C value (measured in mmol/L). Serum glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lipid profile were evaluated using routine standard enzymatic methods with the Roche Cobas 8000 c 702 device.
The Homeostasis Model Assessment (HOMA-IR), triglyceride/HDL-C ratio (TG/HDL-C), and triglyceride-glucose index (TyG) were used to evaluate insulin resistance. HOMA-IR was calculated by multiplying the fasting insulin concentration (µU/mL) by the fasting glucose concentration (mg/dL) and dividing by a constant value of 405 (18). The TG/HDL-C ratio was calculated by dividing the fasting triglyceride levels (mmol/L) by the HDL-C levels (mmol/L) (19). The TyG index was determined using the formula Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] (20).
The Toshiba Alpio 500 ultrasound system was used to examine all patients. A comparative analysis was conducted on the outcomes of patients, distinguishing between those diagnosed with hepatosteatosis and those without. The liver was categorized into three grades based on echogenicity observations during ultrasound imaging: Grade I (mild), grade II (moderate), and grade III (marked) hepatosteatosis (5).
3.1. Statistical Analysis
The statistical analyses were conducted using the Statistical Package for the Social Sciences, version 21.0 (IBM Inc., Chicago, Illinois, USA). The normality of data distribution was evaluated using the Kolmogorov-Smirnov test. Continuous variables were displayed as either the mean ± standard deviation or the median (25th to 75th percentile), depending on their respective distributions. Categorical variables were analyzed using descriptive statistics, reported as frequencies and percentages.
The Student t-test was utilized to compare continuous variables that adhered to a normal distribution across two groups, while one-way analysis of variance (ANOVA) was employed to compare the same variables across three or more groups. The Mann-Whitney U test or the Kruskal-Wallis test was used to analyze continuous variables that deviated from a normal distribution. The chi-square test was used to assess categorical variables. Correlations between MHR and other factors were assessed using Spearman or Pearson correlation analysis.
Receiver operating characteristic (ROC) analysis was used to evaluate the area under the curve (AUC), cutoff value, sensitivity, and specificity of laboratory measures in assessing the MHR for individuals with and without MAFLD. Binomial logistic regression analysis was utilized to examine the relationship between variables and MAFLD. A significance level of less than 0.05 was employed to establish statistical significance.
4. Results
Two hundred eleven children and adolescents with obesity (67 boys and 144 girls) were included in the study. Metabolic associated fatty liver disease (MAFLD) was found in 121 patients (57.3%). Of these, 77 patients (63.7%) had grade 1 hepatosteatosis, 35 patients (28.9%) had grade 2 hepatosteatosis, and 9 patients (7.4%) had grade 3 hepatosteatosis. Comparing the MAFLD group and the simple obesity group, MAFLD was more common in boys than in girls (79.1% vs. 47.2%, P < 0.001). No statistically significant differences were observed between the two groups in terms of age (P = 0.186) and pubertal stage (P = 0.185).
The MAFLD group exhibited significantly elevated levels of AST, ALT, fasting insulin, triglyceride, HOMA-IR, TG/HDL-C, and TyG compared to the simple obesity group (all P < 0.05). The level of HDL-C was significantly lower in the MAFLD group than in the simple obesity group (P = 0.001). No significant differences were found between the two groups in terms of weight SDS, height SDS, and BMI SDS (all P > 0.05). Additionally, levels of fasting blood glucose (FBG), HbA1c, total cholesterol, and LDL-C did not show any differences between the two groups (all P > 0.05).
We compared MHR, NLR, PLR, and LMR levels in children and adolescents with obesity, both with and without MAFLD. The Monocyte/HDL-C ratio was significantly higher in the MAFLD group than in the simple obesity group (0.56 ± 0.19 vs. 0.46 ± 0.14, P < 0.001). However, no significant differences were found in NLR, PLR, or LMR between the two groups (all P > 0.05). Table 1 summarizes the demographic, anthropometric, and clinical features of the study group.
| Variables | Total (n = 211) | Simple Obesity (n = 90) | MAFLD (n = 121) | P-Value |
|---|---|---|---|---|
| Age (y) | 13.8 (10.3 - 15.5) | 13.2 (9.76 - 15.4) | 13.9 (11.3 - 15.6) | 0.186 |
| Male/female | 67/144 (31.8./68.2) | 14/76 (15.6/84.4) | 53/68 (43.8/56.2) | < 0.001 |
| Prepubertal/pubertal | 34/177 (16.1/83.9) | 11/79 (12.2/87.8) | 23/98 (19.0/81.0) | 0.185 |
| Weight SDS | 3.13 ± 1.05 | 2.94 ± 0.94 | 3.27 ± 1.11 | 0.025 |
| Height SDS | 0.35 ± 1.15 | 0.20 ± 1.15 | 0.45 ± 1.14 | 0.117 |
| BMI SDS | 2.85 ± 066 | 2.75 ± 0.66 | 2.93 ± 0.65 | 0.047 |
| ALT (IU/L) | 21.0 (15.0 - 34.0) | 17.0 (13.0 - 23.0) | 28.0 (18.0 - 40.0) | < 0.001 |
| AST (IU/L) | 20.5 (17.0 - 28.0) | 19.0 (17.0-23.8) | 25.0 (18.0-31.3) | < 0.001 |
| FBG (mg/dl) | 92.54 ± 8.44 | 91.64 ± 7.79 | 93.23 ± 8.88 | 0.181 |
| HbA1c (%) | 5.49 ± 0.37 | 5.44 ± 0.47 | 5.52 ± 0.28 | 0.163 |
| Fasting insulin (IU/mL) | 25.8 (15.5 - 35.2) | 22.4 (14.6 - 31.0) | 29.0 (18.9 - 40.0) | 0.001 |
| HOMA-IR | 5.80 (3.48 - 8.15) | 4.76 (3.20 - 6.93) | 6.64 (4.44 - 9.05) | 0.001 |
| TG/HDL | 2.67 (1.86 - 3.53) | 2.21(1.72 - 2.93) | 2.85(2.01 - 3.98) | 0.001 |
| TyG | 8.58 ± 0.40 | 8.50 ± 0.36 | 8.63 ± 0.43 | 0.019 |
| Total cholesterol (mmol/L) | 4.22 ± 0.76 | 4.17 ± 0.73 | 4.26 ± 0.78 | 0.393 |
| Triglyceride (mmol/L) | 1.42 ± 0.58 | 1.30 ± 0.47 | 1.50 ± 0.63 | 0.012 |
| HDL-C (mmol/L) | 1.19 ± 0.26 | 1.26 ± 0.26 | 1.14 ± 0.24 | < 0.001 |
| LDL-C (mmol/L) | 2.40 ± 0.69 | 2.36 ± 0.72 | 2.44 ± 0.67 | 0.393 |
| Neutrophil (×109/L) | 4.54 ± 1.31 | 4.38 ± 1.14 | 4.66 ± 1.42 | 0.155 |
| Lymphocyte (×109/L) | 2.99 ± 0.86 | 2.84 ± 0.72 | 3.09 ± 0.94 | 0.045 |
| Monocyte (×109/L) | 0.59 ± 0.16 | 0.56 ± 0.13 | 0.62 ± 0.18 | 0.013 |
| Platelet (×109/L) | 337 ± 67 | 328 ± 65 | 343 ± 68 | 0.129 |
| MHR (×109/mmol) | 0.52 ± 0.17 | 0.46 ± 0.14 | 0.56 ± 0.19 | < 0.001 |
| NLR | 1.63 ± 0.65 | 1.62 ± 0.51 | 1.63 ± 0.73 | 0.871 |
| PLR | 121.4 ± 41.4 | 121.1 ± 34.0 | 121.6 ± 46.4 | 0.941 |
| LMR | 5.30 ± 1.64 | 5.27 ± 1.34 | 5.32 ± 1.84 | 0.844 |
Abbreviations: MAFLD, metabolic associated fatty liver disease; SDS, standard deviation score; BMI, Body Mass Index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; TG/HDL, triglyceride/HDL-C ratio; TyG, triglyceride-glucose Index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; MHR, monocyte to high-density lipoprotein cholesterol ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio. MHR was significantly higher in the NAFLD group than in the non-NAFLD group.
a Values are expressed as No. (%) or mean ± SD.
A summary of the correlation analysis between MHR and other parameters is presented in Table 2. There was a positive correlation between MHR and fasting insulin (P = 0.011, r = 0.184), HOMA-IR (P = 0.029, r = 0.159), TG/HDL-C (P < 0.001, r = 0.374), TyG (P = 0.005, r = 0.203), triglyceride (P < 0.001, r = 0.257), and the grade of hepatosteatosis (P < 0.001, r = 0.272).
| Variables | R | P-Value |
|---|---|---|
| Age | 0.075 | 0.302 |
| Weight SDS | - 0.015 | 0.841 |
| BMI SDS | 0.005 | 0.941 |
| ALT | 0.099 | 0.170 |
| AST | 0.016 | 0.830 |
| FBG | - 0.070 | 0.337 |
| Fasting insulin | 0.184 | 0.011 |
| HbA1c | - 0.065 | 0.402 |
| HOMA-IR | 0.159 | 0.029 |
| TG/HDL | 0.374 | < 0.001 |
| TyG | 0.203 | 0.005 |
| Total cholesterol | - 0.122 | 0.093 |
| Triglyceride | 0.257 | < 0.001 |
| LDL-C | - 0.016 | 0.831 |
| Grade of hepatosteatosis | 0.272 | < 0.001 |
Abbreviations: SDS, standard deviation score; BMI, Body Mass Index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance.
a Correlation analysis performed between the MHR and age, weight SDS, BMI SDS, fasting blood glucose (FBG), ALT, AST, fasting insulin, HbA1c, HOMA-IR, TG/HDL, TyG, total cholesterol, triglyceride, LDL-C and grade of hepatosteatosis. MHR was positively correlated with fasting insulin, HOMA-IR, triglyceride, and grade of hepatosteatosis.
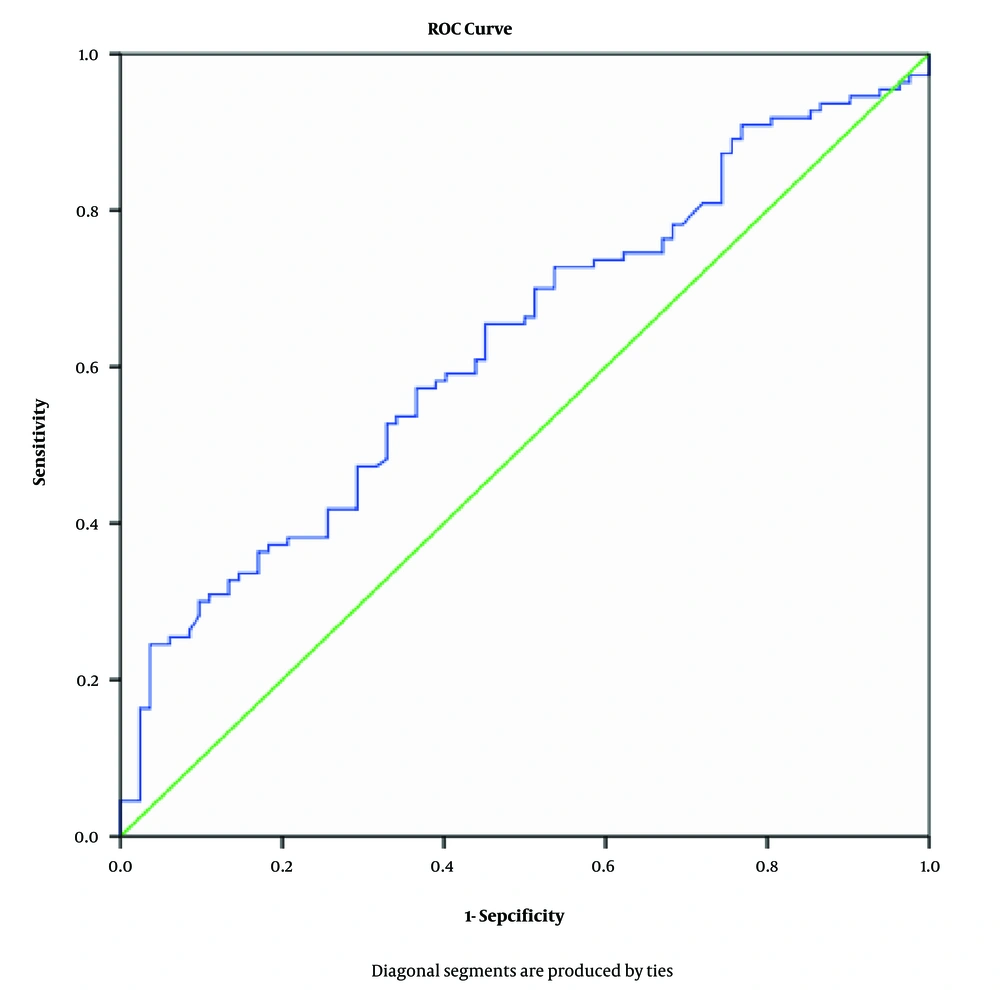
Based on the ROC curve, the cut-off value for MHR to predict MAFLD in children and adolescents with obesity was found to be 0.43 (× 109/mmol), with a sensitivity of 75.45% and a specificity of 46.34%. The area under the curve (AUC) of MHR for distinguishing between those with and without MAFLD was 0.656 (95% CI: 0.550 - 0.707, P = 0.002). The ROC curve for MHR predicting MAFLD is shown in Figure 1.
Receiver operating characteristic (ROC) curve for monocyte-to-high-density lipoprotein cholesterol ratio (MHR) to predict metabolic-associated fatty liver disease (MAFLD)The cut-off value for MHR to predict MAFLD was 0.43 (AUC: 0.656, sensitivity 75.45%, and specificity 46.34%, respectively).
We performed multivariate logistic regression analyses to investigate the association between each variable and MAFLD. There were strong links between having MAFLD and being male (OR: 3.825; 95% CI = 1.673 - 8.740; P = 0.001), having a high ALT level (OR: 1.035; 95% CI = 1.004 - 1.070; P = 0.025), and having a high MHR level (OR: 16.166; 95% CI = 1.408 - 185.610; P = 0.025) (Table 3). Considering confounding factors, MHR persisted as the most significantly associated factor with an elevated risk of MAFLD.
| Variable | Β | SE | OR | 95% CI | P-Value |
|---|---|---|---|---|---|
| Age | 0.028 | 0.063 | 1.028 | 0.908 - 1.160 | 0.663 |
| Gender (1 = male, 0 = female) | 1.341 | 0.421 | 3.825 | 1.673 - 8.740 | 0.001 |
| BMI SDS | 0.508 | 0.318 | 1.661 | 0.891 - 3.100 | 0.111 |
| FBG (mg/dL) | 0.016 | 0.025 | 1.016 | 0.969 - 1.070 | 0.505 |
| ALT (IU/L) | 0.034 | 0.015 | 1.035 | 1.004 - 1.070 | 0.025 |
| AST (IU/L) | 0.004 | 0013 | 1.075 | 0.978 - 1.030 | 0.786 |
| HOMA-IR | 0.072 | 0.057 | 0.989 | 0.962 - 1.200 | 0.202 |
| TG/HDL | -0.011 | 0.267 | 1.365 | 0.587 - 1.670 | 0.968 |
| TyG | 0.311 | 0.959 | 1.004 | 0.208 - 8.940 | 0.745 |
| MHR (×109/mmol) | 2.783 | 1.245 | 16.166 | 1.408 - 185.610 | 0.025 |
Abbreviations: MAFLD, metabolic associated fatty liver disease; BMI, Body Mass Index; SDS, standard deviation score; FBG, fasting blood glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA-IR, homeostatic model assessment of insulin resistance; TG/HDL, triglyceride/HDL-C ratio; TyG, triglyceride-glucose Index; MHR, monocyte to high-density lipoprotein cholesterol ratio. β, standardized regression coefficient; SE, standard error of partial regression coefficient; OR, odds ratio; CI, confidence interval.
a Adjusted R2 = 0.216, P = <0.001.
b The model adjusted was for age, gender, BMI SDS, FBG, ALT, AST, HOMA-IR, TG/HDL, and TyG. High MHR was significantly related to risk of MALFD.
5. Discussion
This retrospective study is the first to examine the relationship between MHR and MAFLD in children and adolescents with obesity. In this population, we observed a significant increase in MHR in those with MAFLD compared to those without; this increase correlated significantly with fasting insulin, triglyceride, HOMA-IR, TG/HDL-C, TyG, and the grade of hepatosteatosis. Our study provides evidence that MHR is closely associated with MAFLD, establishing MHR as an independent risk factor for this condition.
The significantly higher MHR value in the MAFLD group suggests an increase in inflammatory biomarkers. Recent studies in adults have shown that MHR is a non-invasive biomarker for predicting NAFLD, aligning with our results (8, 13-15). Therefore, high MHR values, which are prognostic markers in atherosclerotic diseases, should be carefully evaluated. An analysis of 409 patients from Turkey revealed that individuals with NAFLD had significantly higher MHR than healthy controls. Additionally, age, ALT, and HOMA-IR values exhibited a positive correlation with MHR (14). Similarly, our study found a positive correlation between MHR and fasting insulin, triglyceride, HOMA-IR, TG/HDL-C, and TyG. We also demonstrated that MHR correlated positively with the grade of hepatosteatosis.
Our findings indicate a significant relationship between male gender, elevated ALT levels, high MHR, and MAFLD. Huang et al. demonstrated that in a Chinese population of 14,189 individuals, those with high MHR values were significantly more likely to have NAFLD. Their multivariable logistic regression analysis indicated that MHR is significantly and positively associated with NAFLD risk (15). Furthermore, our study found a link between male gender and an increased risk of NAFLD. A 2015 meta-analysis investigating the incidence of NAFLD in both normal-weight and obese teenagers revealed that the prevalence was twice as high in males, particularly in the obese subgroup. Our findings align with this meta-analysis, indicating that there was a 3.8-fold increase in the risk of NAFLD in males (3).
In a large-scale study of the American population, there was a positive and independent link between NAFLD, the risk of liver fibrosis, and MHR, as determined using FibroScan. This study found that each unit increase in MHR was associated with a 1.87-fold increase in NAFLD risk (13). In our study, each incremental unit increase in MHR was associated with a significant 16.166-fold increase in the risk of MAFLD. Zhao et al. found that MHR has good clinical performance in diagnosing NAFLD, with a cut-off value of 3.434 (× 108/mmol) (8). In our study, the cut-off value of MHR for diagnosing MAFLD in children and adolescents was 0.43 (× 109/mmol). Additionally, Zhao et al. suggested that a diagnostic model combining MHR with ALT, AST, triglyceride, total cholesterol, creatinine, fasting blood sugar, uric acid, and BMI could better predict NAFLD, with an AUC value of 0.931. They proposed that MHR itself or in combination with other parameters could be used to predict NAFLD (8).
Yozgat et al. reported a significant relationship between MHR and insulin resistance (14). Consistent with this, the elevated levels of HOMA-IR, TG/HDL-C, and TyG in our study, which were hypothesized to be predictors of insulin resistance in the NAFLD cohort, validate the association between NAFLD and insulin resistance (19, 20). Our findings support the positive correlation between MHR and TG/HDL-C, TyG, and HOMA-IR. As reported in previous studies, MHR is an easy, cost-effective, and predictive marker for identifying MAFLD in children and adolescents (8, 13-15).
The pathogenesis of obesity involves interactions between adipocytes and other inflammatory cells, including monocytes (21). It has been demonstrated that inflammatory cytokines and inflammation significantly contribute to the development of hepatosteatosis in this interaction (6, 7, 9). Hepatosteatosis occurs through metabolic processes and immune phenotypes resulting from interactions between the liver and other organs. Monocyte-derived macrophages are innate immune cells found in all organs (9). In adipose tissue, the expression of monocyte chemotactic protein 1 (MCP-1) is effective in monocyte recruitment and inflammation stimulation (22). Through the secretion of inflammatory and non-inflammatory factors, liver macrophages and Kupffer cells contribute to the advancement of steatohepatitis and hepatosteatosis in individuals with obesity (10, 23). Depending on microenvironmental conditions, different macrophage populations exhibit pro-inflammatory and repair phenotypic characteristics. With simple steatosis, steatohepatitis is the primary indicator of inflammatory phenotype lipotoxicity. Cytokines secreted from active Kupffer cells inhibit genes that play a role in the lipid metabolism of hepatocytes, thereby increasing hepatosteatosis. Macrophages that play a key role in the progression or decline of hepatosteatosis are also being studied as therapeutic targets (9). Evidence of this is the pharmacological inhibition of monocytes, insulin resistance, liver inflammation, and the alleviation of fibrosis (24).
On the other hand, HDL-C, recognized for its anti-inflammatory properties, inhibits the activation of monocytes and the growth and specialization of monocyte precursor cells (10). High-density lipoprotein cholesterol has been demonstrated to inhibit the synthesis of MCP-1, a molecule crucial for monocyte migration (25). High-density lipoprotein cholesterol inhibits the oxidation of LDL and also opposes the pro-inflammatory and pro-oxidant actions of monocytes. Given the relationship between monocytes and HDL-C in inflammatory processes, MHR is considered an appropriate marker (10). The association between MHR and conditions such as polycystic ovary syndrome (26), metabolic syndrome (27), diabetic retinopathy (28), diabetic nephropathy (29), and mortality from cardiovascular disease (10) has been demonstrated in prior research. Furthermore, our study revealed that children and adolescents diagnosed with MAFLD exhibited elevated concentrations of triglycerides and decreased levels of HDL-C compared to those without MAFLD. This finding provides further evidence that metabolic disorders play a significant role in the development of MAFLD (6).
In children and adolescents with obesity, while MHR is a good biomarker to distinguish NAFLD, there was no association between NLR, LMR, PLR, and NAFLD. The literature presents conflicting reports about NLR, PLR, and LMR in NAFLD (7, 8, 30-33). In a meta-analysis of adult studies, NLR was found to be a promising biomarker in predicting NAFLD and fibrosis (30). On the other hand, Kara et al. found no association between NLR and hepatic inflammation or the severity of fibrosis, arguing that this could be related to other metabolic disorders present in patients (31). Kohsari et al. reported that LMR is associated with NAFLD and Fatty Liver Index (33). Zhou et al. identified a nonlinear correlation among NLR, PLR, and NAFLD (32). In line with our study, Duan et al. showed that the NLR, PLR, and LMR are not different for people with obesity and NAFLD compared to only obesity. However, within the same investigation, levels of inflammatory cytokines, including IL-1β, IL-17, and IL-6, were notably elevated in comparison to possible biomarkers (7). In the study of Zhao et al. mentioned earlier, healthy controls showed higher MHR, NLR, and LMR than individuals with NAFLD, while PLR was fairly lower. Moreover, according to ROC analysis, MHR predicted NAFLD above other inflammatory biomarkers (8). The differences in findings in the literature may be attributed to variations in sample sizes, population age groups, and whether control groups included individuals with obesity.
5.1. Limitation
There were some limitations to our study: (1) we used ultrasound rather than liver biopsy or fibroscan to diagnose hepatosteatosis; (2) since our study is retrospective, a judgment on the cause-and-effect relationship between MHR and MAFLD could not be reached; (3) there was no healthy control group without obesity; and (4) other biomarkers were not considered to assess inflammation. To verify the results of this study, further research is needed, involving larger sample groups with healthy controls, utilizing more accurate diagnostic methods for MAFLD, and evaluating other inflammation markers.
5.2. Conclusions
As a result, MHR significantly increases in pediatric MAFLD and correlates with the grade of hepatosteatosis. The monocyte/HDL-C ratio, as a novel inflammatory biomarker, can be used to predict MAFLD in children and adolescents with obesity.