1. Background
An upper endoscope is introduced into the second section of the duodenum during endoscopic retrograde cholangiopancreatography (ERCP), a combined endoscopic and fluoroscopic procedure that allows other instruments to enter through the major duodenal papilla and into the pancreatic and biliary ducts. When necessary, the injection of contrast material into these ducts enables radiologic visualization and therapeutic interventions (1).
When ERCP first came into existence in 1968, it was immediately recognized as a direct and safe method of assessing pancreaticobiliary illness (2). Initially developed as a diagnostic technique to evaluate pancreaticobiliary system disorders, ERCP has evolved into a primarily therapeutic modality due to advances in endoscope design, imaging technology, and the development of catheters and tools (3). Using a regular duodenoscope, Waye reported the first successful ERCP in 1976 on a 3.5-month-old infant with cholestasis. Although ERCP was not widely used in pediatric populations initially, its use has increased significantly since the late 1980s with the development of smaller diameter duodenoscopes and accessories (4).
Diagnostic ERCP has limited applications (apart from pancreaticobiliary manometry for acute relapsing pancreatitis and in some patients with type II sphincter of Oddi dysfunction), and CT, magnetic resonance cholangiopancreatography (MRCP), and EUS imaging have supplanted diagnostic testing. However, ERCP remains a standard procedure for treating bile duct leaks, choledocholithiasis, and the palliation of malignant obstructive jaundice. Furthermore, ERCP is now a viable option for pancreatic endotherapy for strictures, stones, and other pancreatic duct leak symptoms (5).
There are few pediatric indications for ERCP, and not many pediatric patients are included in published studies. Therefore, biliopancreatic diseases are uncommon indications for this method's use. Due to insufficient experience, limited information regarding the method's safety, and infrequent indications in children and infants, ERCP is used cautiously (6). While the indications for ERCP in children and adolescents differ, they are similar in cases related to liver transplantation, cancer, and choledocholithiasis. On the other hand, neonatal cholestasis diagnostic workup and suspected pancreaticobiliary maljunction have been reported as the primary indications for ERCP in infants (7).
Endoscopic retrograde cholangiopancreatography is one of the endoscopic procedures with the highest rate of complications (8). Although earlier studies have indicated that ERCP is safe for pediatric patients, concerns about the procedure's safety and outcomes for young patients still exist due to the small number of documented cases in the literature (9). The three most frequently documented side effects in children are bleeding, infection, and post-ERCP pancreatitis. However, the incidence of complications appears to differ across case series, possibly influenced by various factors such as the operator, patient selection, and underlying illness (10).
2. Objectives
Considering the limitations of previous studies regarding the indications and complications of ERCP in children, the current research aimed to determine the causes of ERCP in children, the frequency of related complications, and their relationship with the cause and type of intervention performed on patients hospitalized in Namazi and Abu Ali Sina Hospitals.
3. Methods
This cross-sectional study was undertaken between September 2022 and December 2023 at Namazi and Abu Ali Sina Hospitals. The study included all pediatric patients aged 1 to 18 years who underwent ERCP. Patients with a history of previous gastrointestinal surgery, such as gastric or duodenal resection that could influence the ERCP operation or its results, were not allowed to participate in the research. Patients with severe cardiovascular illness or coagulopathy or those with other major comorbidities that increased the likelihood of anesthetic or procedural problems were also excluded. To ensure the accuracy and reliability of the results, participants who lacked pertinent medical records or other essential data were not included in the cohort. In our study, patient selection for ERCP strictly adhered to the guidelines outlined in the "pediatric liver and gastrointestinal disease" book.
The study design and protocol underwent approval and monitoring by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1402.130). Before their inclusion in the study, written informed consent was obtained from the parents or legal guardians of all participants. This study followed the principles described in the Helsinki Declaration, which sets the ethical requirements for medical research involving human participants. All procedures involving human participants were conducted with strict adherence to ethical principles, prioritizing the participants' rights, safety, and well-being.
The indications for conducting ERCP in children at these facilities were determined according to the guidelines outlined in the "pediatric liver and gastrointestinal disease" book as follows (11): Biliary atresia versus neonatal hepatitis, Alagille syndrome and paucity syndrome, congenital hepatic fibrosis, Caroli disease and Caroli syndrome, biliary strictures, choledochal cyst, treatment of pancreas divisum, diagnosis of annular pancreas, cystic dilation of the pancreatic duct (pancreatocele), treatment of benign biliary strictures, removal of bile duct stones, treatment of biliary complications after liver transplantation, diagnosis and treatment of primary sclerosing cholangitis, biliary obstruction due to parasitic infestation (parasitic infestation: Ascaris), diagnosis and treatment of sphincter of Oddi dysfunction, diagnosis and treatment of pancreatic trauma, treatment of acquired immune deficiency syndrome cholangiopathy, treatment of chronic pancreatitis, and drainage of pancreatic pseudocysts.
The medical records were reviewed for the following variables: Age, gender, indication for ERCP, findings of ERCP, therapeutic interventions, pre-procedural imaging such as ultrasound and MRCP, associated surgical interventions, and specific laboratory values prior to ERCP (including liver function tests and serum pancreatic enzyme measurements). Early (within 72 hours) and late (within 6 months) procedural complications were documented by checking hospital records and conducting follow-up examinations within 1 - 6 months post-surgery. The failure rate of the procedure was determined by calculating the percentage of patients who did not achieve the desired clinical goal, such as biliary decompression and remission of pancreatitis, after undergoing the procedure. To evaluate complications within 72 hours and up to 6 months after ERCP, patients underwent daily visits for the first three days post-procedure, followed by monthly follow-up visits. Additionally, relevant data were reviewed from medical records.
All information about the included patients was entered into SPSS software (version 26, IBM Corporation, Armonk, NY) and analyzed. To report qualitative data, the mean ± standard deviation criterion was used. Frequencies and percentages were used to report descriptive statistics for categorical variables (e.g., gender and indication of ERCP). We conducted Fisher's exact test to analyze the relationship between indications and complications. This statistical test was chosen due to its suitability for contingency tables, particularly in situations with small sample sizes. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Demographic Characteristics and Laboratory Data
From September 2022 to December 2023, 100 ERCPs were performed on 56 children, with 53 (53%) performed at Namazi Hospital and 47 (47%) at Abu Ali Sina Hospital. Fifty-three-point-six percent (53.6%, N = 30) of the patients were female, and the average age of the studied population (mean ± standard deviation [SD]) was 10.22 ± 4.80 years. Table 1 presents a summary of the laboratory data obtained from the patients.
| Parameters | Mean ± SD | Minimum - Maximum |
|---|---|---|
| WBC (cells/μL) | 8.41 ± 4.12 | 2 - 22 |
| Hb (g/dL) | 11.10 ± 2.11 | 7 - 16 |
| Plt (K/μL) | 245.35 ± 148.42 | 9 - 650 |
| Total protein (g/dL) | 7.37 ± 3.69 | 4 - 26 |
| Albumin (g/dL) | 3.84 ± 0.63 | 3 - 6 |
| Globulin (g/dL) | 2.89 ± 0.81 | 2 - 5 |
| AST (U/L) | 384.33 ± 386.68 | 8 - 1700 |
| ALT (U/L) | 429 ± 452.52 | 4 - 1420 |
| Alkp (U/L) | 691.09 ± 498.94 | 120 - 2840 |
| Total bilirubin (mg/dL) | 5.09 ± 9.46 | 0 - 41 |
| Direct bilirubin (mg/dL) | 1.19 ± 1.58 | 0 - 7 |
| Amylase (U/L) | 209.29 ± 317.79 | 38 - 1190 |
| Lipase (U/L) | 360.33 ± 508.499 | 5 - 1430 |
| TG (mg/dL) | 135.33 ± 71.02 | 88 - 217 |
| Cholesterol (mg/dL) | 155.00 ± 53.73 | 122 - 217 |
| HDL (mg/dL) | 49.50 ± 33.23 | 26 - 73 |
| LDL (mg/dL) | 106.50 ± 62.93 | 62 - 151 |
| ESR (mm/h) | 36.33 ± 26.00 | 12 - 74 |
| CRP (mg/L) | 35.89 ± 48.19 | 1 - 100 |
| GGT (U/L) | 312.33 ± 226.82 | 132 - 567 |
4.2. MRCP and Sonography Findings
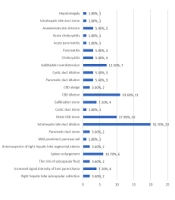
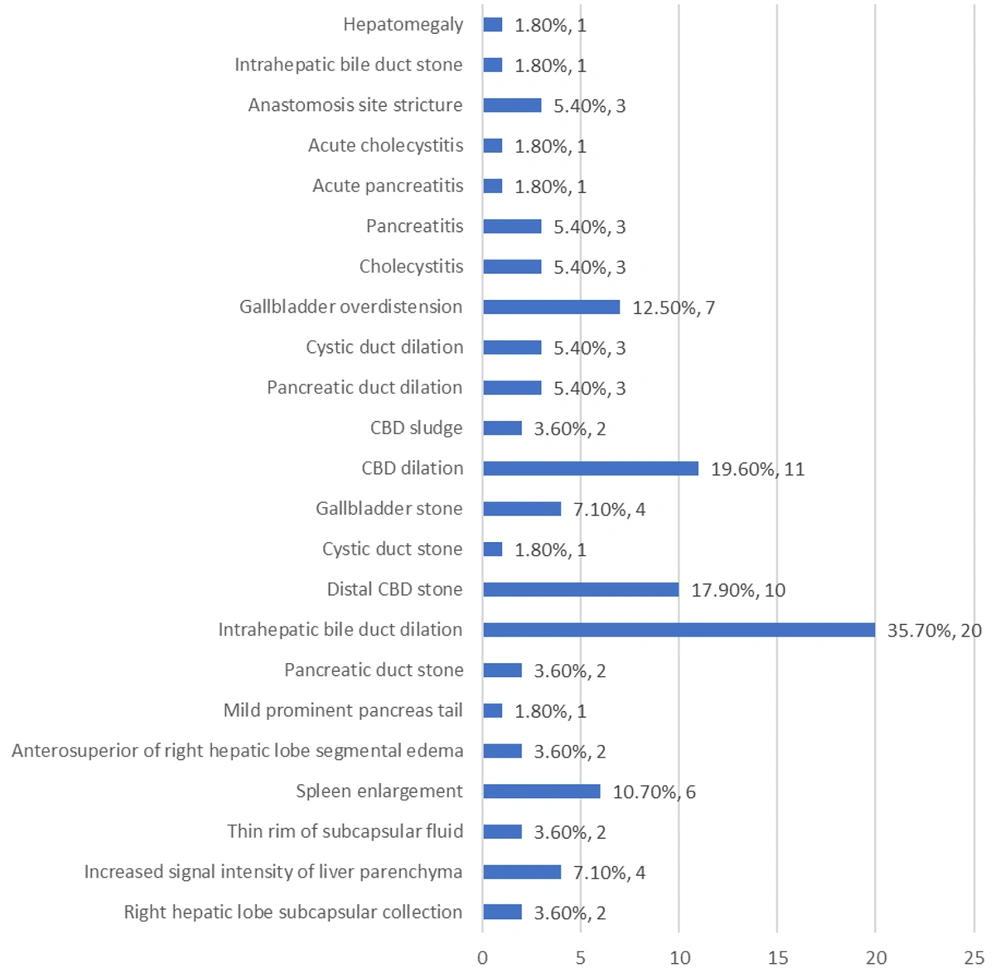
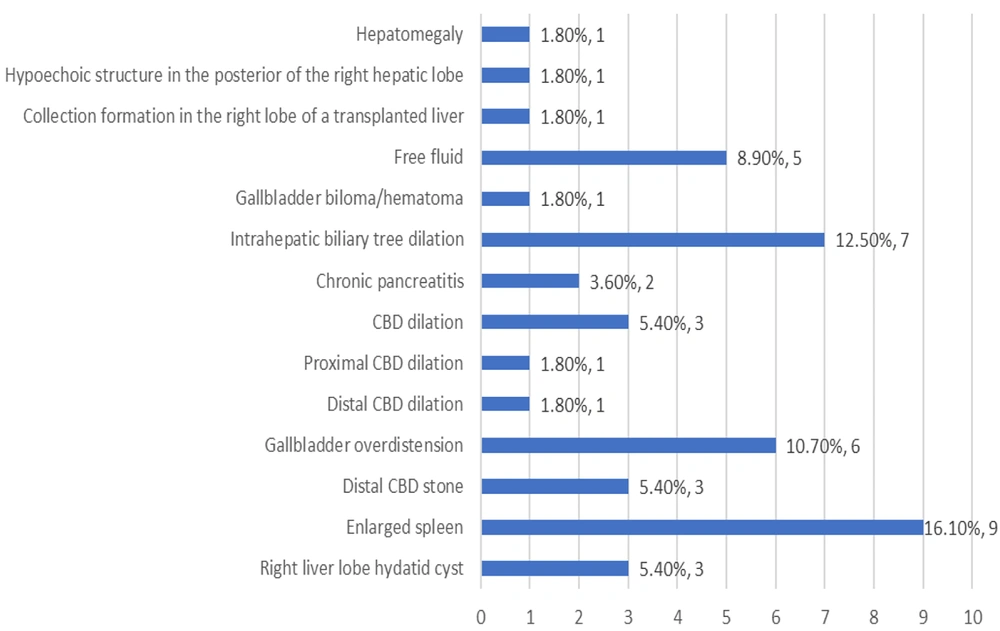
As depicted in Table 2 and Figure 1, the most frequently observed findings in MRCP were intrahepatic bile duct dilation (35.7%), dilation of the common bile duct (CBD) (19.6%), and the presence of a distal CBD stone (17.9%). Similarly, the most common findings in sonography were an enlarged spleen (16.1%), dilation of the intrahepatic biliary tree (12.5%), and overdistension of the gallbladder (10.7%) (Table 3 and Figure 2).
| Variables | Number of Subjects (%) |
|---|---|
| Right hepatic lobe subcapsular collection | 2 (3.6) |
| Increased signal intensity of liver parenchyma | 4 (7.1) |
| Thin rim of subcapsular fluid | 2 (3.6) |
| Spleen enlargement | 6 (10.7) |
| Anterosuperior of right hepatic lobe segmental edema | 2 (3.6) |
| Mild prominent pancreas tail | 1 (1.8) |
| Pancreatic duct stone | 2 (3.6) |
| Intrahepatic bile duct dilation | 20 (35.7) |
| Distal CBD stone | 10 (17.9) |
| Cystic duct stone | 1 (1.8) |
| Gallbladder stone | 4 (7.1) |
| CBD dilation | 11 (19.6) |
| CBD sludge | 2 (3.6) |
| Pancreatic duct dilation | 3 (5.4) |
| Cystic duct dilation | 3 (5.4) |
| Gallbladder overdistension | 7 (12.5) |
| cholecystitis | 3 (5.4) |
| pancreatitis | 3 (5.4) |
| Acute pancreatitis | 1 (1.8) |
| Acute cholecystitis | 1 (1.8) |
| Anastomosis site stricture | 3 (5.4) |
| Intrahepatic bile duct stone | 1 (1.8) |
| Hepatomegaly | 1 (1.8) |
| Variables | Number of Subjects (%) |
|---|---|
| Right liver lobe hydatid cyst | 3 (5.4) |
| Enlarged spleen | 9 (16.1) |
| Distal CBD stone | 3 (5.4) |
| Gallbladder overdistension | 6 (10.7) |
| Distal CBD dilation | 1 (1.8) |
| Proximal CBD dilation | 1(1.8) |
| CBD dilation | 3 (5.4) |
| Chronic pancreatitis | 2 (3.6) |
| Intrahepatic biliary tree dilation | 7 (12.5) |
| Gallbladder biloma/hematoma | 1 (1.8) |
| Free fluid | 5 (8.9) |
| Collection formation in the right lobe of a transplanted liver | 1 (1.8) |
| Hypoechoic structure in the posterior of the right hepatic lobe | 1 (1.8) |
| Hepatomegaly | 1 (1.8) |
4.3. ERCP Indications, Complications and Failure
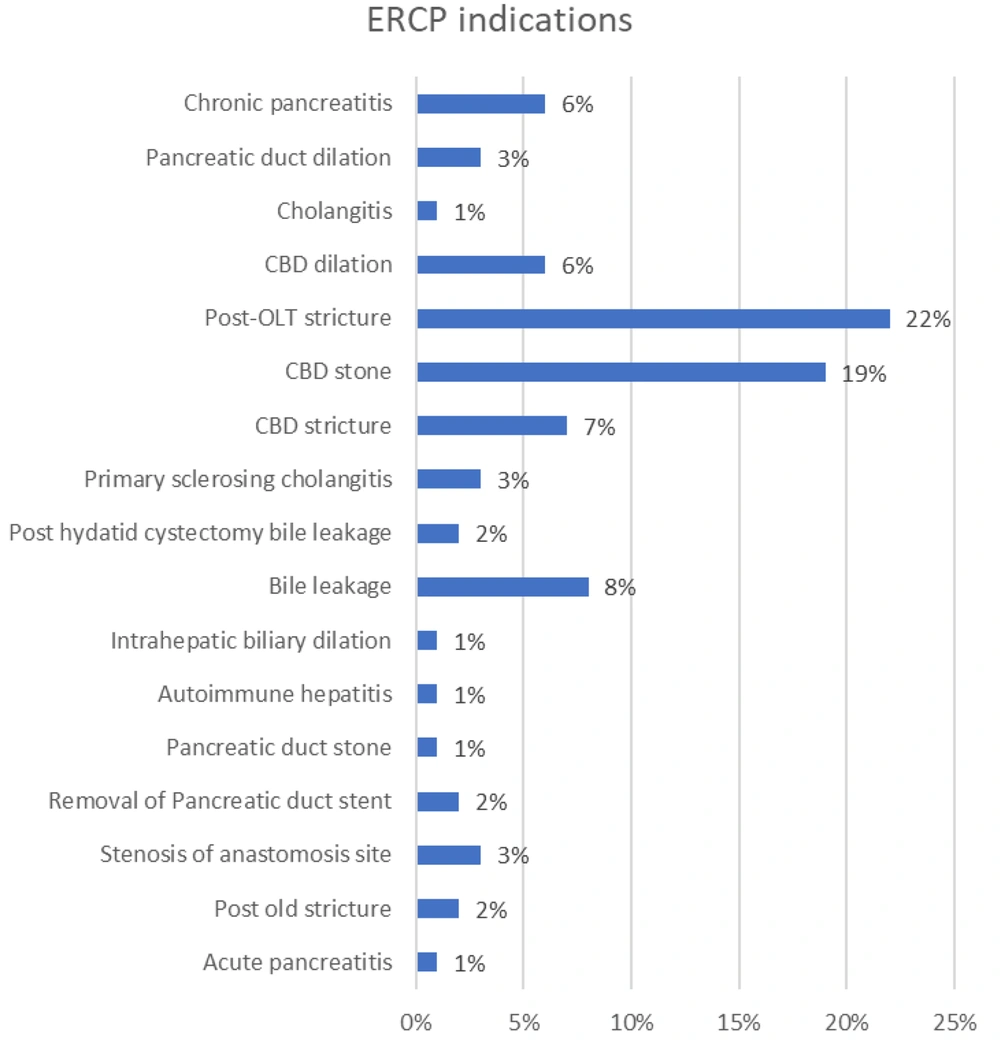
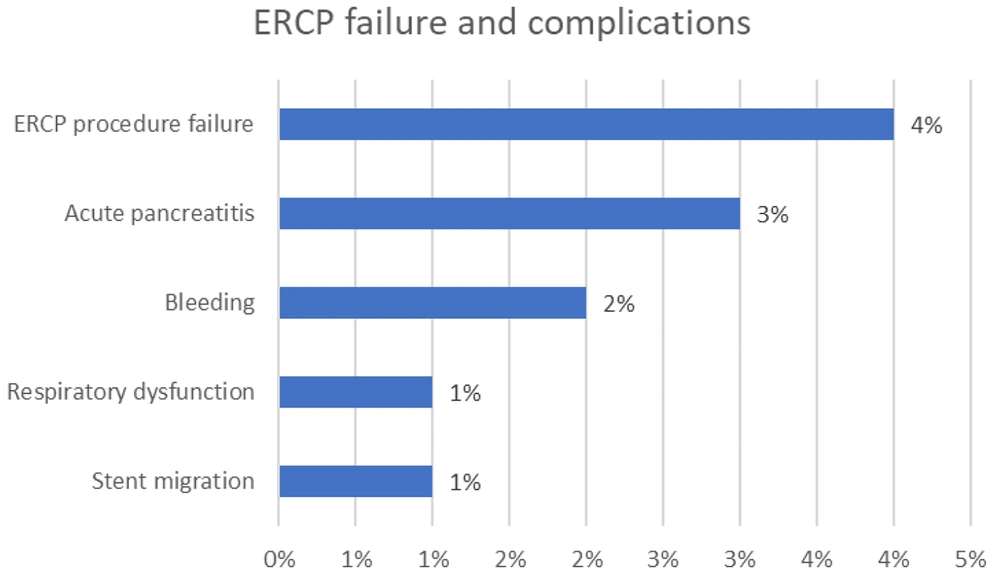
As presented in Table 4 and Figure 3, the most common indications for ERCP were post-orthotopic liver transplantation (OLT) stricture (22%), CBD stone (19%), and bile leakage (8%). The most prevalent complications observed were acute pancreatitis (3%) and bleeding (2%). One patient experienced stent migration (1%), and another had respiratory dysfunction (1%), necessitating the stopping of the ERCP procedure. Additionally, in 4 ERCPs (4%), patients experienced procedure failure (Table 5 and Figure 4). A Fisher's exact test was conducted to assess the relationship between complications and indications. The calculated p-value exceeded 0.05, leading us to reject the null hypothesis, indicating insufficient evidence to establish a correlation between any indications and complications.
| Variables | Number of ERCPs (%) |
|---|---|
| Acute pancreatitis | 1 (1) |
| Post old stricture | 2 (2) |
| Stenosis of anastomosis site | 3 (3) |
| Removal of pancreatic duct stent | 2 (3) |
| Pancreatic duct stone | 1 (1) |
| Autoimmune hepatitis | 1(1) |
| Intrahepatic biliary dilation | 1 (1) |
| Bile leakage | 8 (8) |
| Post hydatid cystectomy bile leakage | 2 (2) |
| Primary sclerosing cholangitis | 3 (3) |
| CBD stricture | 7 (7) |
| CBD stone | 19 (19) |
| Post-OLT stricture | 22 (22) |
| CBD dilation | 6 (6) |
| Cholangitis | 1 (1) |
| Pancreatic duct dilation | 3 (3) |
| Chronic pancreatitis | 6 (6) |
| Complications | Number of ERCPs (%) |
|---|---|
| Acute pancreatitis | 3 (3) |
| Bleeding | 2 (2) |
| Stent migration | 1 (1) |
| Respiratory dysfunction | 1 (1) |
| Total | 7 (7) |
| ERCP procedure failure | 4 (4) |
4.4. Therapeutic Interventions in Pediatric ERCP
During the ERCP procedures, a range of therapeutic interventions were employed to address biliary and pancreatic disorders in pediatric patients. These interventions included:
- Sphincterotomy: Incision of the sphincter of Oddi to facilitate access to the bile or pancreatic ducts.
- Stone extraction: Techniques such as balloon extraction, basket retrieval, or mechanical lithotripsy were utilized to remove stones or other obstructions from the ducts.
- Stent placement: Insertion of stents (plastic or metal tubes) into the ducts to relieve obstruction, promote drainage, or prevent stricture formation.
- Biopsy: Tissue sampling from the duct walls for histological analysis, particularly in cases of suspected malignancy or inflammatory conditions.
- Balloon dilation: Dilation of strictures or narrowings in the bile or pancreatic ducts using balloon dilation techniques.
- Injection therapy: Injection of medications or substances into the ducts to manage bleeding, tumor obstruction, or sphincter of Oddi dysfunction.
- Laser therapy: Ablation of strictures, tumors, or other lesions within the ducts using laser therapy techniques.
These therapeutic interventions aimed to alleviate obstruction, manage complications, and improve overall clinical outcomes in pediatric patients undergoing ERCP.
5. Discussion
Due to its primarily therapeutic nature, ERCP necessitates specialized infrastructure and a multidisciplinary team of well-trained personnel. Establishing an efficient and up-to-date infrastructure and cultivating a competent and skilled group of endoscopists would undoubtedly impose significant costs on the healthcare system of any nation. This could potentially discourage the progress of advanced ERCP services in developing countries (12). This study represents a novel Iranian investigation that provides data on ERCP indications and complications in pediatrics. The study collected demographics, laboratory data, MRCP, and sonographic findings of children who underwent ERCP, making it unique in this regard.
A total of 100 ERCP procedures were identified during the one-year study period. The study population had a mean age of 10.22 ± 4.80 years, indicating a younger age compared to a similar research study conducted in the USA (13) and an older age compared to another study conducted in the Czech Republic (6). The disparity in the average age of pediatric patients receiving ERCP in different countries might be attributed to several factors, including differences in disease prevalence and incidence across regions, varying treatment guidelines, disparities in healthcare infrastructure and access, cultural influences on healthcare-seeking behaviors, distinct diagnostic practices and technology availability, and variations in genetic and environmental factors.
In the present study, there was a nearly equal representation of boys and girls who underwent ERCP, which aligns with the gender distribution observed in studies conducted by Giefer and Kozarek (9) and Ugurlu (14). The predominant sonographic finding in the ongoing inquiry was splenomegaly, which is frequently observed in children suffering from chronic liver disease (15). Given that almost 50% of ERCPs were performed at a liver transplant center, this observation was expected.
The most prevalent observation in MRCP was the dilation of the intrahepatic bile duct, which also ranked among the most frequently observed findings in sonography. Previous studies have noted that patients with severe diffuse liver disease may not exhibit significant intrahepatic biliary dilation, or it may be mild, even when extrahepatic obstructive disease is present. In simple terms, imaging techniques may not accurately assess the seriousness of the blockage, which can be considered a limitation of MRCP (16). Hence, the actual incidence of intrahepatic bile duct dilation is anticipated to exceed even the estimated value reported in the MRCP investigation.
The most frequent reasons for performing ERCP in this study were post-OLT stricture (22%), CBD stone (19%), and bile leakage (8%). The occurrence of these results was predictable, as the most common imitator of biliary complications after transplantation is the recurrence of the primary disease (17). The identification of these complications should be conducted using sophisticated endoscopic procedures. In a previous meta-analysis conducted by Hosseini et al., it was found that the most frequent reasons for performing ERCP in pediatric patients were related to the biliary system, which aligns with the current findings (7). Furthermore, a separate investigation carried out by Asenov et al. found that CBD stones and postoperative problems were the prevailing reasons for performing ERCP in pediatric patients, consistent with the findings of the current study (18).
In another study conducted by Perera et al., it was found that pancreatic disorders were the predominant reason for performing pediatric ERCP (19). In a separate study conducted by Keane et al., pancreatitis and biliary obstruction emerged as the prevailing indications (20). The differences in research findings between our study and the mentioned studies regarding the most frequent reasons for performing ERCP in pediatric patients can be attributed to a combination of variations in the population, differences in research methods, disparities in healthcare practices, changes over time, patterns of patient referrals, advancements in diagnostic techniques, and potential biases in the publication of research results.
The current study observed that the most common complication was acute pancreatitis, occurring in 3% of ERCPs. Several previous investigations have similarly identified pancreatitis as the most prevalent complication (4, 19, 21, 22). However, the prevalence of this complication in the current study was lower than that reported in previous investigations. Post-ERCP pancreatitis is believed to be caused by elevated pressure in the duct of Wirsung, resulting from inflammation near the ampulla produced by the use of instruments during ERCP (23).
Our study reported a 4% failure rate in ERCPs, consistent with previous pediatric investigations that generally reported failure rates below 10% (6, 19, 21, 24-26). Nevertheless, the meta-analysis conducted by Sun et al. in 2022 revealed a failure rate of 26%, which was notably higher than the rates recorded in our study and other similar research. However, the meta-analysis encompassed studies published until February 2022, potentially including earlier research conducted in settings with less advanced infrastructure and by operators with lower levels of expertise (27).
In the study by Lorio et al., conducted between January 2004 and January 2021, a total of 287 pediatric patients underwent 716 ERCP procedures at academic centers in the USA. The operating success rate was notably high at 95.5%, and there were no reported deaths during this period. The pediatric ERCP adverse event rate stood at 12.7%, highlighting a significant incidence of complications within this patient cohort. Younger age was linked to increased case complexity, a higher rate of adverse events, and a greater likelihood of requiring repeat ERCP procedures. Case complexity scores correlated with prolonged operation times and a higher occurrence of adverse events. Stent removal and pancreatic stenting often preceded adverse events. Conditions like pancreatitis, pancreatic divisum, and pancreatic stenosis were associated with a higher incidence of adverse events and a higher rate of repeat ERCP procedures in pediatric patients. The study concluded that pediatric ERCP carries a higher adverse event rate compared to adults, and the complexity grading system proposed by Cotton et al. (as cited by Lorio et al.) is applicable to pediatric patients (24).
The present investigation found no statistically significant relationship between any of the indications and complications associated with ERCP. The occurrence of certain complications is anticipated to have a significant association with the indication of the procedure, as the duration of the procedure may vary depending on the indication. Prior research has indicated that the duration of ERCP is closely associated with certain complications, such as pancreatitis (28). Hence, further research with a larger sample size is required to determine the relationship between indications and complications.
The current investigation faced a few limitations. An intrinsic limitation of this study was its cross-sectional design, which could potentially introduce biases, poor recording, or missing information in the medical records. Moreover, our hospitals are subspecialty centers for pediatric liver transplantation, which may have led to a selection bias toward pre- or post-OLT cases. Finally, we missed long-term complications and recurrence rates due to the lack of follow-up. Future prospective cohort studies are required to uncover pediatric ERCP cure rates, as well as long-term complications.
To comprehensively address the observed differences in indications, complications, and failure rates compared to other studies, we need to delve deeper into several factors that could contribute to these variations.
- Patient population: Variations in patient demographics, such as age distribution, underlying conditions, and disease prevalence, can significantly impact ERCP indications and outcomes. Studies conducted in different regions or healthcare settings may have distinct patient populations with varying disease profiles, leading to differences in procedural indications and complication rates.
- Healthcare practices and guidelines: Discrepancies in healthcare practices and adherence to clinical guidelines across different institutions or countries can influence ERCP utilization and patient outcomes. Variations in diagnostic criteria, treatment protocols, and procedural thresholds may contribute to differences in indications and complication rates observed between studies.
- Operator expertise and experience: The proficiency and experience of endoscopists performing ERCP play a crucial role in procedural success and complication rates. Variations in operator skill levels, procedural techniques, and adherence to safety protocols can contribute to differences in outcomes across studies. Centers with highly experienced endoscopists may achieve lower complication rates and higher procedural success rates compared to those with less experienced operators.
- Technology and infrastructure: Disparities in healthcare infrastructure, availability of advanced endoscopic equipment, and support services can impact ERCP outcomes. Centers with state-of-the-art facilities, including high-definition endoscopes, advanced imaging modalities, and comprehensive peri-procedural care, may achieve better outcomes and lower complication rates compared to those with limited resources.
- Case selection and referral patterns: Variations in case selection criteria and referral patterns across different centers or studies can influence the spectrum of cases undergoing ERCP and subsequent outcomes. Centers specializing in certain conditions or procedures may receive referrals for more complex cases, leading to differences in complication rates and procedural success rates compared to more general centers.
- Study design and methodology: Variations in study design, including retrospective vs. prospective design, sample size, inclusion criteria, follow-up period, and data collection methods, can influence the interpretation of results and comparisons between studies. Studies with larger sample sizes, prospective designs, longer follow-up periods, and rigorous data collection methods may provide more reliable estimates of complication rates and procedural outcomes compared to smaller or retrospective studies.
5.1. Conclusions
In summary, this research offers valuable insights into the indications and outcomes of ERCP among pediatric patients in Iran. While many aspects of our findings align with global patterns, such as the high prevalence of complications like post-ERCP acute pancreatitis, distinct demographic and procedural characteristics emerge when compared to other studies. The study's cross-sectional methodology, combined with its distinctive inclusion criteria and inherent limitations, underscores the need for meticulous interpretation. The absence of a conclusive correlation between ERCP indications and subsequent complications highlights a substantial gap that necessitates additional comprehensive investigation. It is crucial to consistently enhance our understanding of ERCP procedures, considering their growing complexity and significance. This is especially important in view of the broader healthcare consequences and challenges encountered in different regions.