1. Background
Childhood growth disorder, a condition indicating developmental delays in height and weight compared to healthy peers, serves as a common sign across various disorders (1-3). While not classified as a disease, it acts as a shared indicator among numerous health challenges. Recognizing growth status as a pivotal marker for health and nutritional well-being underscores the impact of unfavorable environmental conditions on an individual's development (4-6). Effective management of growth disorders necessitates a focused consideration of its risk factors, categorizing them into non-organic and organic factors.
Non-organic factors predominantly involve issues such as insufficient nutrition, reduced appetite, and inadequate maternal awareness regarding essential child nutrition principles (7-9). On the other hand, organic factors encompass chronic or acute illnesses causing interference with nutrient absorption or metabolism (8-10). The World Health Organization estimates that approximately 60% of the 11 million deaths in children under five are attributed to malnutrition and highlights its significant impact, particularly in developing countries (11). Malnutrition-related diseases not only lead to increased complications but also result in prolonged hospitalization, elevated mortality risk, and higher treatment costs (12).
Conditions associated with inflammation or atrophy in the proximal small intestine, such as celiac disease, can further complicate the health of malnourished individuals. Mucosal atrophy in the intestine is identified as a consequence of malnutrition, affecting enteropancreatic stimulation and impairing liver and pancreas function (8, 13-15). Notably, the enterokinase enzyme is secreted through the intestinal mucosa and its decreased level may reduce the activation of other pancreatic enzymes (16).
2. Objectives
As recognizing malnutrition as a serious global health concern emphasizes the urgent need for the development of innovative therapeutic approaches, this study aimed to investigate the therapeutic impact of pancreatic enzymes (PERT) on enhancing growth indicators in children experiencing malnutrition, aligning with the World Health Organization's classification.
3. Methods
3.1. Patients and Settings
The present study is a single-blind randomized clinical trial conducted at the gastroenterology clinic of 17 Shahrivar Hospital, Iran. Given that the researcher and patients might be aware of the intervention types, only the data analyzer was kept blinded to the study. The inclusion criteria were children aged 2 to 14 years with growth disorders, characterized by a Z-score for weight below -2 based on the World Health Organization guidelines (17), and no organic issues such as cardiac, pulmonary, endocrine, metabolic, renal disorders, or malabsorption disorders, particularly those with elastase less than 200 and steatorrhea. Patients using pancreatic enzymes (Creon) or those unable to adhere to nutritional recommendations and drug supplements, or who experienced significant adverse effects deemed unsuitable for continuation by the gastroenterologist, were excluded. Due to the limited evidence, this pilot study was conducted on 30 subjects in each group.
3.2. Randomization
A nurse, not involved in the study, enrolled participants, and the sequence allocation was performed by a statistician using relevant software. To ensure balance and concealment, blocked randomization with block sizes of four was employed, utilizing SAS version 9 for the randomization process. Children were randomized into two groups, PERT and control, based on these random blocks.
3.3. Intervention
All patients received a uniform nutritional plan and identical mineral supplements, including iron, zinc, and vitamin D, to address malnutrition over 2 months. The PERT group received a 2-month course of 1000 U/kg pancreatic enzymes (Abbott, Germany) with each of the three main meals. The control group received the same mineral supplements and dietary regimen. Routine treatment included daily zinc supplementation at 3.0 mg/kg body weight, up to a maximum of 6 mg, using Zinc Plus syrup (Eurovital), and weekly iron supplementation using ferrous sulfate syrup (Abidi Pharmaceutical Company) at 25 mg for ages 2 - 5 and 45 mg for ages 5 and above. Vitamin D was administered at 600 units daily using vitamin D ultra 1000 drops (Vita Biotechnics). Caloric requirements were adjusted based on a nutritionist’s recommendation, providing an additional 300 to 500 calories tailored to each patient's condition. During the intervention period, patients were monitored by a gastroenterologist after two months, and parents were advised against altering their children's dietary habits.
3.4. Data Gathering
Patients' demographic characteristics and anthropometric indices—including height, weight, body mass index (BMI), and Z-scores—were measured at baseline and after the intervention (2 months). Weight was measured on a standardized scale (Seca, Germany) with an accuracy of 0.1 kg. Height was measured without shoes, standing against a wall, using a calibrated meter (Seca, Germany) with an accuracy of 0.1 cm. BMI was calculated as weight in kilograms divided by the square of height in meters. Z-scores for weight, height, and BMI were determined using the Medscape calculator.
Clinical tests performed at baseline included a complete blood count (CBC), electrolytes, vitamin D levels, kidney and liver function tests, C-reactive protein (CRP), iron, ferritin, total protein, albumin, serum immunoglobulin A (IgA), anti-tissue transglutaminase antibodies, complete urine analysis, urine culture, thyroid function tests, stool analysis, and measurements of elastase and calprotectin in stool.
- Primary Outcome: Weight gain.
- Secondary Outcomes: Changes in height, BMI, and Z-scores.
3.5. Ethical Considerations
After obtaining written informed consent from the patients, they were enrolled in the study at no cost for any stage of treatment. The costs of the drug provision were covered by the researcher. The ethics committee of Guilan University of Medical Sciences approved the study (number: IR.GUMS.REC.1402.087), and it was registered at the Iranian Registry of Clinical Trials (IRCT20230313057703N1).
3.6. Statistical Analysis
Descriptive statistics, including number, percent, means, and standard deviation, were employed. The normality of quantitative data was assessed using the Kolmogorov-Smirnov test. For normally distributed data, t-tests were conducted, and for non-normally distributed data, the Mann-Whitney U test was utilized. Differences in measures were calculated at the beginning and end of the study. Qualitative variables were compared using the Chi-Square test and Fisher's exact test, with a significance level set at a P-value < 0.05.
4. Results
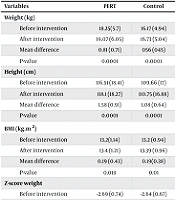
This study involved 60 children, with 30 in each of the PERT and control groups. Table 1 indicates no statistically significant differences between the groups in terms of sex and age (P = 0.292 and P = 0.063, respectively). In the PERT group, significant differences were observed before and after the intervention in weight, height, BMI, weight Z-score, and height Z-score (P < 0.05). Similarly, the control group showed significant changes in weight, height, BMI, weight Z-score, height Z-score, and BMI Z-score before and after the intervention (P < 0.05). However, there were no significant differences between the groups in terms of the mean differences in weight, BMI, and the Z-scores (P > 0.05). The only significant difference was in height growth, where the mean difference before and after intervention was significantly higher in the PERT group compared to the controls (Table 2).
| Variables | Pancreatic Enzyme Replacement Therapy; No. (%) | Control; No. (%) | P-Value |
|---|---|---|---|
| Sex | 0.0292 | ||
| Male | 20 (66.7) | 16 (53.3) | |
| Female | 10 (33.3) | 14 (46.7) | 0.063 |
| Age (y) | |||
| 2 - 8 | 15 (50) | 22 (73.3) | |
| 8 - 14 | 15 (50) | 8 (26.7) |
| Variables | PERT | Control | P-Value |
|---|---|---|---|
| Weight (kg) | 0.108 | ||
| Before intervention | 18.25 (5.7) | 16.17 (4.94) | |
| After intervention | 19.07 (6.05) | 16.73 (5.04) | |
| Mean difference | 0.81 (0.71) | 056 (045) | |
| P-value | 0.0001 | 0.0001 | |
| Height (cm) | 0.018 | ||
| Before intervention | 116.51 (18.41) | 109.66 (17) | |
| After intervention | 118.1 (18.27) | 110.75 (16.88) | |
| Mean difference | 1.58 (0.91) | 1.08 (0.64) | |
| P-value | 0.0001 | 0.0001 | |
| BMI (kg.m2) | 0.964 | ||
| Before intervention | 13.2(1.14) | 13.2 (0.94) | |
| After intervention | 13.4 (1.21) | 13.39 (0.96) | |
| Mean difference | 0.19 (0.43) | 0.19 (0.38) | |
| P-value | 0.019 | 0.01 | |
| Z-score weight | 0.408 | ||
| Before intervention | -2.69 (0.74) | -2.64 (0.67) | |
| After intervention | -2.33 (0.77) | -2.18 (0.55) | |
| Mean difference | 0.35 (0.22) | 0.28 (0.43) | |
| P-value | <0.0001 | 0.01 | |
| Z-score height | 0.126 | ||
| Before intervention | -1.5 (1.03) | -1.3 (0.17) | |
| After intervention | -1.2 (1.08) | -1.07 (0.71) | |
| Mean difference | 0.29 (0.2) | 0.22 (0.15) | |
| P-value | 0.001 | 0.001 | |
| Z-score BMI | 0.691 | ||
| Before intervention | -2.7 (1.88) | -2.6 (1.51) | |
| After intervention | -2.51 (2.06) | -2.36 (0.98) | |
| Mean difference | 0.18 (0.51) | 0.24 (0.58) | |
| P-value | 0.18 | 0.03 |
5. Discussion
The therapeutic role of PERT in addressing malnutrition in children with conditions related to exocrine pancreatic insufficiency (EPI), such as cystic fibrosis, has been acknowledged (18). It has been demonstrated that PERT in such conditions provides effective outcomes in reducing gastrointestinal symptoms, improving nutrient absorption by addressing digestive disturbances, consequently enhancing nutritional status, and improving the quality of life for patients (19). Our study aimed to investigate the impact of PERT on growth indicators in malnourished children without exocrine pancreatic disorders, following the World Health Organization classification. The intragroup analysis in both groups showed that all variables except the BMI Z-score in the PERT group significantly changed before and after the intervention. However, intergroup analysis indicated that only height in the PERT group was significantly higher than the control group.
Our findings revealed no statistically significant association between gender distribution and age categories of the studied children in terms of growth indicators across the two treatment groups. This lack of significant correlation aligns with existing literature (20), supporting the notion that PERT may not exert a distinct influence based on gender or age categories.
Moreover, our investigation regarding weight-related parameters indicated a significant increase in weight after PERT intervention in the treatment group. However, no significant difference in the weight change of malnourished children was observed between the PERT and control groups. This finding is consistent with a study by Bartels et al. in which children with acute malnutrition receiving PERT showed shorter hospital stays and lower mortality risk, but no significant difference in weight gain compared to the control group (10). The lack of significant differences in weight change between PERT and control groups may be attributed to the relatively short treatment duration, suggesting that more conclusive results might be achieved in longer-term intervention periods.
In terms of height, our study demonstrated a statistically significant increase in the average height of malnourished children in the PERT treatment group compared to the control group. This finding aligns with Schulze-Frenking et al., who reported a positive impact of PERT on height in children with Mucopolysaccharidosis type II, suggesting that PERT may beneficially influence height in malnourished children (21).
Regarding BMI, while our results indicated a significant increase in the average BMI after PERT intervention in the treatment group, no significant difference in BMI change was observed between the malnourished children in the PERT and control groups. This outcome is consistent with Guven et al., who found that although PERT effectively increased the BMI of malnourished children, it did not significantly differ from the control group (13). This suggests that PERT may not have a distinct effect on BMI compared to routine treatment.
Additionally, our examination of BMI Z-Score revealed no statistically significant difference in the average BMI Z-Score change between the malnourished children in the PERT and control groups. Although the control group showed a significant increase in BMI Z-Score, the PERT group did not exhibit a significant change. Guven et al. also reported similar findings, where the increase in the average BMI of malnourished children in the PERT group compared to the control group was not statistically significant (13). These findings indicate that, despite increases in height and weight, the effect of PERT on BMI Z-Score may not significantly differ from routine treatment.
This pilot study successfully evaluated malnourished children without underlying diseases. However, several limitations should be noted. The follow-up duration was only two months, which might not capture all effects of the intervention. Additionally, the study's pilot nature and limited sample size might affect the generalizability of the findings. We assessed only healthy individuals, suggesting that future research could explore the effects of this treatment on patients with diverse diseases to aid clinicians in managing malnutrition more effectively. Therefore, larger, multicenter studies with varied doses of PERT and longer follow-up periods are recommended.
5.1. Conclusions
This study provides insights into the therapeutic effects of PERT on growth indicators in malnourished children. While PERT significantly improved height and weight, it did not show significantly different outcomes in BMI Z-Score compared to routine treatment. This highlights the complex factors influencing growth in malnourished children and underscores the need for extended research to explore these dynamics further, considering underlying conditions.