1. Background
Tetralogy of Fallot (ToF) is known as the most prevalent cyanotic congenital heart defect (1).
According to diverse clinical presentations, many patients may have insufficient pulmonary blood flow following the right ventricular outflow tract stenosis (2).
In these patterns, patent ductus arterious (PDA) or Aortopulmonary collateral arteries take blood to one of the left or right pulmonary artery branches or to some defined parts of the lung to enhance the arterial oxygen content (3).
It seems that postponing the repair of these congenital defects may contribute to progressive hypoxic situations and further collateral developments (4).
One of the most challenging characteristics in patients with ToF is the presence of major aortopulmonary collateral arteries (MAPCAs), which are reported between 3 to 5 percent of all ToF patients (5).
Furthermore, the blood flow in the MAPCAs is accompanied by some critical situations, especially in extra-corporeal circulation and critical care after the surgery (6).
Suppose no evidence relies on collaterals in patients with ToF and remains open during and after the surgery due to the undiagnosed ones. In that case, it may result in catastrophic events after the surgery (7).
It is worth mentioning that the undiagnosed open MAPCA caused perfusion disturbances during cardiopulmonary bypass and also affected pulmonary perfusion during and after the surgery (5).
In the meantime, transthoracic echocardiography is one of the cardiac evaluation procedures done before ToF surgery. Other procedures such as cardiac CT scan, cardiac MRI, and cardiac catheterization are beneficial alternatives (8).
Some diagnostic procedures seem to result in various findings without superiority, and taking an appropriate approach to evaluate ToF patients before surgery remains debated. Introducing a diagnostic gold standard for these patients may be reasonable to minimize the adverse clinical outcomes of intra-operative and postoperative patients.
2. Objectives
We presented clinical outcomes after the surgery of ToF patients who had open MAPCA and were undiagnosed with transthoracic echocardiographic findings.
3. Methods
3.1. Study Design and Participants
This retrospective cohort study was conducted on consecutive pediatric patients under two years of age who underwent ToF total correction surgery with the modified transannular patch technique from January 2018 to December 2023 in Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran. The Mashhad University of Medical Sciences ethics committee approved the study protocol with the number of IR.MUMS.REC.1399.082, which complies with the declaration of Helsinki. This study was part of the clinical registry of patients with congenital cardiac defects who underwent cardiac surgery. Informed consent was obtained from the patient's guardians.
Exclusion criteria were congenital heart surgery with complex ToF, patients with preoperative arrhythmias, patients with absent pulmonary valves or pulmonary atresia, patients who needed unifocalization, critical conditions (severe renal and hepatic failure) before surgery, upper airway congenital anomalies, a history of cold agglutinins, parents/guardians not providing written informed consent, and cardiac surgeries.
All of the patients were diagnosed with ToF with trans-thoracic echocardiography and were admitted for a total correction surgery approach. Another pediatric cardiologist confirmed the first echocardiographic findings with a second echocardiography.
In the congenital cyanotic cardiac defects color Doppler transthoracic echocardiography, especially in short-axis and supra-sternal views, we confronted the blood flow in the para-aortic arch, descending aorta, and the origin part of the subclavian artery. Based on the MAPCA presence, we evaluated the continuous waves similar to the PDA pattern, except in velocity and blood circulation. The other differences between PDA and MAPCA Doppler findings are related to the pressure wave amplitudes, which are significantly lower in MAPCA presence than in PDA. According to these findings, we considered patients with high suspicion of the presence of MAPCA.
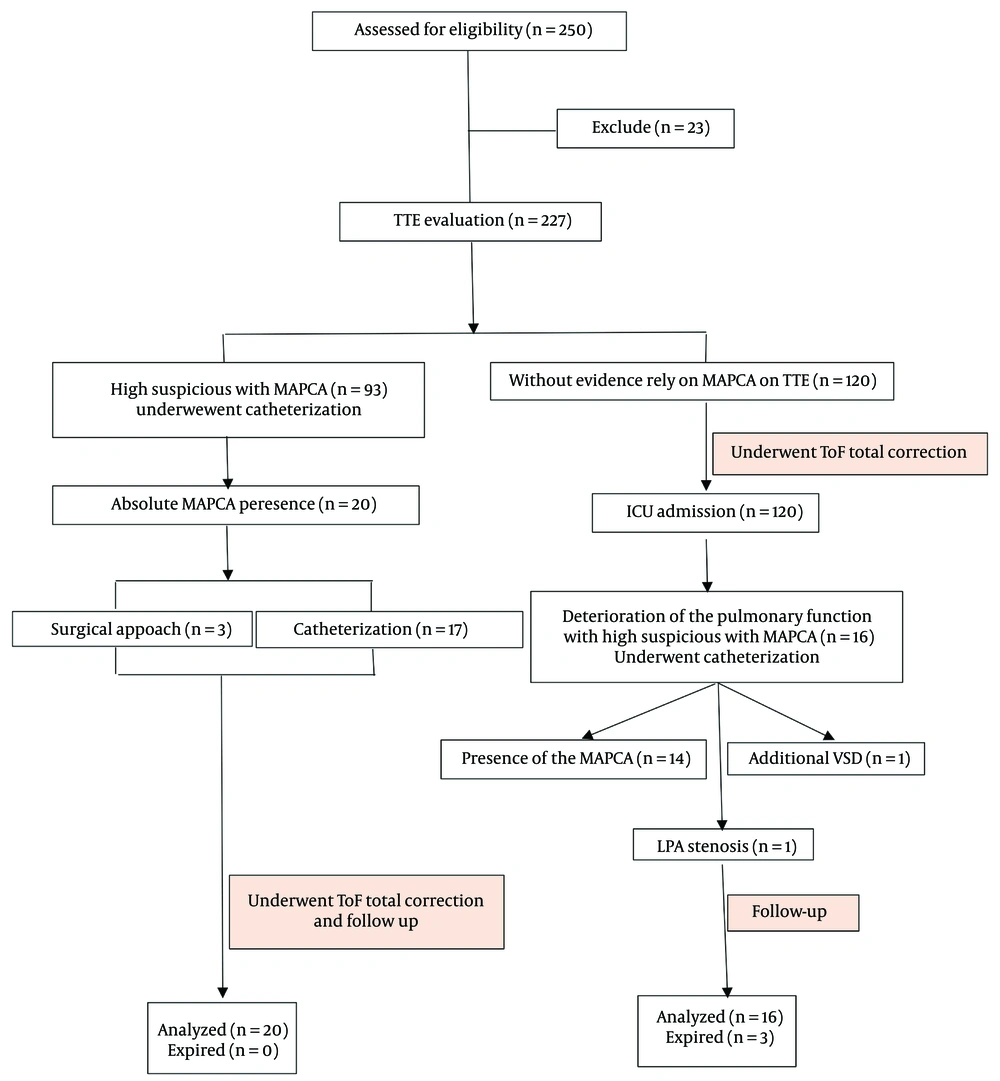
Based on the echocardiographic findings, the patients with high suspicion of the presence of MAPCA underwent cardiac catheterization intending to close the collateral arteries. After that, they underwent ToF total correction surgery if this procedure was successful. Conversely, if the catheterization approach failed, they underwent ToF total correction surgery simultaneously with MAPCA closure.
3.2. Major AortoPulmonary Collateral Arteries Closure Strategy
Major aortopulmonary collateral arteries closure was performed percutaneously with different kinds of PDA closure coils in our hospital: (1) MAPCA closure with Perimembranous-VSD Amplatzer, (2) MAPCA closure with Nit-Occlud PFM coil, (3) MAPCA closure with Gianturco coil, and (4) MAPCA closure with MReye Flipper coil. On the other hand, the patients with no evidence of MAPCA in the echocardiographic findings (as considered undiagnosed patients) underwent ToF total correction surgery (Figure 1).
3.3. Perfusion Strategy
All patients underwent median sternotomy. The CPB was not initiated until the activated coagulated time (ACT) was over 480 seconds, followed by 350 mg/kg of heparin administration. After ensuring this situation, cannulation was performed. Then, the CPB was initiated after confirming favorable conditions by the cardiac surgeon, cardiac anesthesiologist, and perfusionists. For myocardial protection, we used Custodiol® at 30 mL/kg after the aortic cross-clamp as an antegrade route. The oxygenator in all patients was Sorin® (D901 Lilliput 1 Oxygenator for pediatric < 5 kg and D902 Lilliput 2 Oxygenator for pediatric > 5 kg). To prime the CPB circuit, we used isotonic saline 0.9% (700 - 800 mL), human albumin (1 gr/kg), heparin (1 mg/kg), sodium bicarbonate 7.5% (2 mEq/L as 100 mL of prime solution volume), mannitol (500 mg/kg), and methylprednisolone (30 mg/kg).
Non-pulsatile perfusion with a roller pump was recommended. The CPB flow in the mild hypothermic strategy was maintained at 3.5 L/min/m2 (body surface area). During CPB, the alpha-stat strategy was administered to evaluate arterial blood gas. Thus, PaCO2 and PaO2 were maintained at 35 - 45 mmHg and 150 - 250 mmHg, respectively. We used the conventional ultrafiltration (CUF) method for all patients during cardiac surgery.
3.4. Anesthesia Strategy
In the operating room, the anesthetic induction was initiated with ketamine (1 - 2 mg/kg), fentanyl (10 - 15 mcg/kg), midazolam (0.1 mg/kg), and then atracurium (0.2 mg/kg). For anesthesia maintenance, we used propofol at 50 - 70 mcg/kg/min and sufentanil at 0.2 - 0.5 mcg/kg. Normothermia was monitored via a nasopharyngeal thermometer.
After the surgery, only the pediatrics with pulmonary deterioration that compromised mechanical ventilation support, followed by failure in the weaning process of the mechanical ventilation, with high suspicion of the presence of the MAPCA, were referred to the pediatric cardiac catheterization department. If the cardiac catheterization was successful and the MAPCA was closed, the patient entered the ICU and began the weaning process. On the other hand, due to any circumstances, such as difficulty in achieving MAPCA, lack of a suitable landing zone, and the failure to insert the device into the MAPCA, the patients underwent thoracotomy as a surgical approach to close the collateral(s).
So, the three groups of patients were compared in the following clinical outcomes:
3.4.1. Outcomes
- At first, demographic variables (age, gender, and BMI) were recorded. The primary endpoints were: (1) weaning time from mechanical ventilation, and (2) postoperative ICU and hospital stay (from surgery date to discharge from the hospital).
- The secondary outcomes were:
(1) CPB time and cross-clamp time.
(2) Renal function tests: Blood urea nitrogen (BUN), serum creatinine, and glomerular filtration rate (GFR) before the surgery (T1), after first ICU admission (T2), and 24 hours (T3) after ICU re-admission.
(3) Thoracic blood loss (mL/kg) and blood products (packed red blood cells, cryoprecipitate, and platelets) transfusion requirements at the first and second times after ICU entrance.
(4) In-hospital mortality.
3.5. Statistical Methods
Statistical analysis was performed using SPSS software version 26.0 (Chicago, IL, USA). The quantitative results were presented as mean ± SD and median (inter-quartile range = IQR) for normal and non-normally distributed data. Categorical data were expressed as frequency (percentage). Normal distribution of the quantitative data was checked using the Shapiro-Wilks test, Q-Q, and box plots. Also, propensity score matching was done to minimize selection biases. Independent Student's t-test was used for variables with a normal distribution, and the Mann–Whitney test compared non-normal quantitative variables. The homogeneity of categorical variables between the groups was analyzed using the independence chi-square or Fisher's exact test. After checking relevant assumptions, paired quantitative variables were compared with paired sample t-test or Wilcoxon test. The significance level was considered as P <0.05.
4. Results
In this study, 250 eligible patients with a mean age of 13.9 ± 7.6 months who underwent ToF total correction surgery participated. The male patients comprised 68.2% of all patients.
4.1. Baseline Characteristics
The comparison of the patients' demographic variables in Table 1 shows that the two treatment groups in this study had no significant differences in age and BMI (P > 0.05). Only the gender of the patients was heterogeneous between treatment groups (P < 0.001) (Table 1).
| Variables | ToF with MAPCA (Correct Diagnosed with TTE) | ToF Without MAPCA (Misdiagnosed with TEE) | P-Value | |
|---|---|---|---|---|
| Surgical Approach | Catheterization Approach | Surgical Approach | ||
| Age, mo | 14.4 ± 15.3 | 18.12 ± 11.2 | 16.8 ± 13.9 | 0.612 |
| BMI | 14.3 ± 2.4 | 14.9 ± 1.8 | 14.6 ± 2.7 | 0.667 |
| Gender | < 0.001 b | |||
| Male | 32 (68.08) | 51 (61.44) | 89 (74.16) | |
| Female | 15 (31.92) | 32 (38.56) | 31 (25.84) | |
The Demographic Characteristics of Correctly Diagnosed Major Aortopulmonary Collateral Arteries with Trans-Thoracic Echocardiography and Misdiagnosed Patients a
4.2. Primary Outcomes
The analysis of the primary outcomes showed that the ICU stay in patients with a correct diagnosis before the ToF surgery (59.5 ± 13.6 hours) was statistically lower than in misdiagnosed open MAPCA patients who underwent the surgical approach of the ToF group (144.2 ± 23.8 hours) (P < 0.001). Additionally, the hospital stay in patients with a correct diagnosis of MAPCA via TTE was statistically lower than in others (8.7 ± 1.1 days vs. 14.3 ± 3.4 days; respectively, and P < 0.001). The weaning time from mechanical ventilation in patients with a correct diagnosis before the ToF surgery was also statistically lower than in misdiagnosed open MAPCA patients who underwent the surgical approach of the ToF group (4.8 ± 3.2 hours vs. 19.0 ± 11.9 hours; respectively, and P < 0.001).
4.3. Secondary Outcomes
4.3.1. CPB Time and Cross-Clamp Time
The analysis of CPB time revealed that this period was not statistically different between the two groups of patients who underwent cardiopulmonary bypass with the correct diagnosis and those misdiagnosed (65.1 ± 16.8 minutes vs. 71.3 ± 15.6 minutes, respectively, and P = 0.059). Similarly, there were no statistical differences in the cross-clamp time between patients with a correct diagnosis of the presence of MAPCA and a surgical strategy, and the surgical approach group with a misdiagnosed presence of the open MAPCA group (52.7 ± 10.4 minutes vs. 60.1 ± 11.3 minutes; respectively, and P = 0.075).
4.3.2. Renal Function Test
The renal function test analysis showed no statistical differences between patients in all groups before the procedures and after 24 hours from the first ICU admission. However, the investigation of the effect of the study groups and measurement time on creatinine clearance and glomerular filtration variations through GEE showed that these measurements after re-operation of patients who needed closure procedures dramatically plummeted compared to the other groups who did not need re-operation (P = 0.009) (Table 2).
| Renal Function Test and Time | ToF with MAPCA (Correct Diagnosed with TTE) | ToF without MAPCA (Misdiagnosed with TEE) | P-Value | |
|---|---|---|---|---|
| Surgical Approach | Catheterization Approach | |||
| BUN | ||||
| T1 | 24.62 ± 11.49 | 23.84 ± 10.31 | 24.63 ± 6.76 | 0.710 |
| T2 | 25.80 ± 7.31 | 24.61 ± 11.12 | 25.38 ± 9.31 | 0.601 |
| T3 | - | - | 29.02 ± 9.23 | - |
| Serum Creatinine | ||||
| T1 | 0.44 ± 0.10 | 0.38 ± 0.11 | 0.35 ± 0.09 | 0.069 |
| T2 | 0.49 ± 0.10 | 0.41 ± 0.10 | 0.38 ± 0.12 | 0.058 |
| T3 | - | - | 1.02 ± 0.81 | - |
| GFR | ||||
| T1 | 109.71 ± 28.89 | 105.20 ± 31.76 | 113.07 ± 22.85 | 0.074 |
| T2 | 105.42 ± 19.05 | 102.21 ± 12.10 | 109.21 ± 16.15 | 0.120 |
| T3 | - | - | 85.21 ± 12.69 | - |
The Renal Function Tests in Patients with Correctly Diagnosed Major Aortopulmonary Collateral Arteries with Trans-Thoracic Echocardiography and Misdiagnosed
4.3.3. Thoracic Blood Loss and Blood Products Transfusion
In terms of the blood loss through thoracic drains, the analysis of data showed that the mean thoracic blood loss in patients who were undiagnosed for open MAPCA through TTE had no statistical difference with the surgical or catheterization approach with the corrected TTE diagnosis group (2.4 ± 1.5 mL/kg vs. 1.6 ± 1.0 mL/kg; respectively, and P = 0.069).
Additionally, the need for all types of blood products in the misdiagnosed TTE group was higher than in the corrected diagnosed group patients, such as packed RBC (9.1 ± 1.6 mL/kg vs. 4.2 ± 2.2 mL/kg; respectively, and P < 0.001), cryoprecipitate (6.0 ± 0.9 mL/kg vs. 2.9 ± 0.4 mL/kg; respectively, and P < 0.001), and platelets (10.2 ± 1.2 mL/kg vs. 8.1 ± 1.2 mL/kg; respectively, and P < 0.001).
4.3.4. In-hospital Mortality
Unfortunately, we had three mortalities in the patients whose TTE findings were undiagnosed as there was no evidence to rely on the presence of open MAPCA. The first and second patients, after ICU entrance, experienced two failures in mechanical ventilation weaning efforts and thus underwent cardiac catheterization. Due to hemodynamic compromises during this procedure, followed by poor pulmonary clinical functions, the patients expired during catheterization.
The latter one was the patient that, before the cardiac catheterization investigation with the aim of the probable collateral arteries, had expired in the ICU due to ventilator parameters failures.
5. Discussion
The congenital heart defect that affects the cardiopulmonary system as a compensatory effect due to prolonged hypoxic states in ToF patients is MAPCA. This phenomenon develops a systemic-to-pulmonary connection that may originate from the aorta or one of its branches to the pulmonary arterial vasculature (5, 9).
Regarding the nature of this defect, it is patently obvious that the presence of these collateral arteries can be a life-threatening event. Therefore, some clinical modalities facilitated open MAPCA diagnosis and were considered a bridge to treatment (10). In this study, we aimed to show the essential role of cardiopulmonary catheterization for the detection of probable MAPCA in patients before ToF total correction surgery.
The cardiopulmonary bypass strategy in ToF patients with open MAPCA can interfere with the surgeon's vision and may be the leading cause of blood loss during and after the surgery (3). Additionally, surgery involving MAPCA during and after the procedure can lead to hypoperfusion of other organs (11). Although the CPB time and cross-clamp time in patients without evidence of open MAPCA who underwent ToF surgery showed no statistical difference compared to others in our study, the mean time for these clinical parameters was higher than in patients with closed MAPCA after cardiac catheterization or during cardiac surgery.
The higher mean times in these patients may be due to the need for more time to recover the cardiopulmonary system after cardiac arrest during surgery. Additionally, there may be deterioration of cardiac function, simultaneously with pulmonary rehabilitation during CPB weaning (12). Our findings showed that patients who had open MAPCA after ToF surgery required more blood product transfusions. Some studies have found similar results (12, 13).
Moreover, massive blood transfusion in these patients may be associated with longer CPB times and higher surgical blood loss (13). Our findings showed that creatinine clearance and GFR in patients who underwent re-operation to close the MAPCA dramatically plummeted. This may result from hypoperfusion of the kidneys and surgical procedures causing inflammatory storms that have deleterious effects on the organs.
Therefore, comorbidities in these patients may be followed by a higher rate of ICU stay and hospital stay. Our findings indicate that comorbidities after ToF with open MAPCA result in higher ICU and hospital stays (14).
Life-threatening comorbidities following ToF surgeries with open MAPCA also lead to higher mortality rates (15). Other investigations revealed that some lung segments in TOF cases do not have a dual blood supply and are supplied only by the MAPCA source. In such cases, MAPCA should not be closed, but unifocalization should be performed (16, 17).
On the other hand, high-flow vessels can exacerbate pulmonary function after surgery with open MAPCA (18). In patients with dual supply lung segments, pulmonary edema is predictable. Therefore, complications of intraoperative bleeding and postoperative pulmonary edema can be prevented by making the right decision about MAPCA treatment before surgery (19).
This study had limitations, such as a retrospective setting approach and the lack of consideration of potential risk factors for poor outcomes.
5.1. Conclusions
It can be concluded that if ToF patients have occluded MAPCAs, their prognosis is better than that of patients who remain open to MAPCA. Therefore, occluding them percutaneously or during surgical procedures before performing ToF surgery is reasonable. We recommend that complete cardiopulmonary catheterization be considered the critical definitive diagnostic approach for patients with ToF before total correction surgeries.