1. Background
Abnormal uterine bleeding (AUB) is defined as bleeding that originates from the uterine corpus and is abnormal in terms of duration, volume, frequency, and/or regularity (1, 2). It is the most common reason for gynecology-related hospital admissions in adolescence (2). Abnormal uterine bleeding decreases the quality of life, affects school attendance, and limits participation in sports and social activities (3). Abnormal uterine bleeding is commonly observed due to the immaturity of the hypothalamic-pituitary-ovarian (HPO) axis during adolescence. Polycystic ovary syndrome (PCOS), thyroid disorders, and hyperprolactinemia are among the most frequent endocrine causes (4, 5). More than 90% of girls start menstruating before the age of 14. The HPO axis must mature for ovulation and regular menstrual cycles. Maturation of the HPO axis generally occurs between six months and three years after menarche (6). By the fifth year after menarche, only 75% of menstrual cycles are ovulatory. Lack of progesterone secreted by ovarian follicles and excessive E2 production cause anovulation and irregular menstrual bleeding (4, 7, 8). During adolescence, the length of menstrual cycles is between 21 and 45 days. Menstruation usually lasts 2 - 7 days, causing an average of 30 - 40 mL of blood loss, and the use of 3 - 6 pads per day (9). Chronic menstrual bleeding that usually exceeds 80 mL leads to anemia (5). Heavy menstrual bleeding (HMB) is the most common form of AUB and is defined as excessive menstrual bleeding that disrupts the physical, social, emotional, or material quality of life of women (10). Signs of HMB include changing pads more often than every few hours, using double hygiene protection, frequent soiling of clothes or sheets, and the occurrence of blood clots greater than 2.5 cm in diameter (11). The causes of HMB are divided into structural causes such as polyp, adenomyosis, leiomyoma, malignancy, and non-structural causes such as coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not-yet classified causes such as PALM-COEIN (12). Structural causes of HMB are rarely seen in the adolescent age group (1.3 - 1.7%) (13). Anovulation is the most common cause of HMB in adolescence. Von Willebrand disease (VWD), platelet function disorders, thrombocytopenia, and clotting factor deficiencies are the most common bleeding disorders in adolescents presenting with HMB. The prevalence of coagulopathy in adolescents hospitalized due to HMB is reported as 5 - 28% (14-16). The PCOS, which is another cause of anovulatory cycles, should be considered as an underlying cause of AUB since it can be easily overlooked in this age group (17).
2. Objectives
The present study aimed to evaluate the etiological distribution of AUB in adolescents and to compare clinical characteristics and management approaches based on hemoglobin levels. The gap in understanding the relationship between hemoglobin levels and clinical management of AUB in adolescents remains significant.
3. Methods
3.1. Study Design
This retrospective study was conducted at our clinic between January 1, 2018, and December 31, 2021. Study reporting was carried out according to the STROBE guidelines (18). The study protocol was approved by the Local Ethics Committee of Diyarbakır Gazi Yaşargil Training and Research Hospital (approval number: 962 — date: December 31, 2021). The parents of the participants signed an informed consent form according to the Declaration of Helsinki.
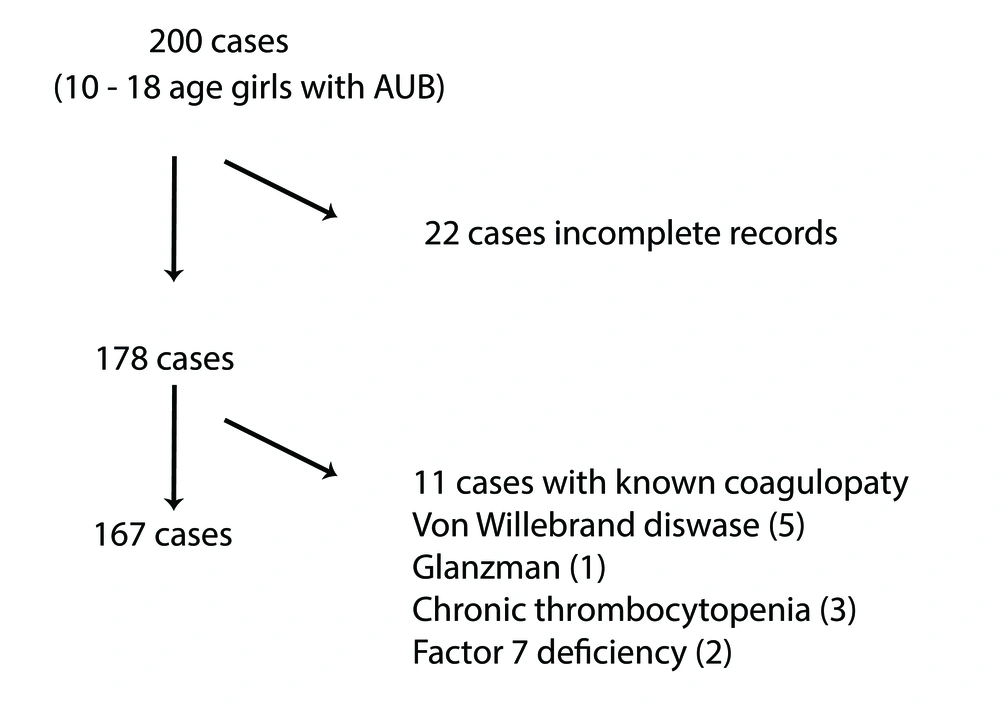
In the study, 167 adolescent female patients between the ages of 10 and 18 years who were diagnosed with AUB were included. The sample size was determined based on hospital admission records from 2018 to 2021, covering a wide range of cases for representativeness.
Inclusion criterion is adolescents aged 10 - 18 years diagnosed with AUB and exclusion criteria included patients with malignancies, hematological diseases, or incomplete records. The inclusion and exclusion process is summarized in Figure 1.
Demographic characteristics, family history, presenting complaints, examination findings, laboratory and radiological evaluation results, data on treatment regimens, and responses to treatment were obtained from the electronic medical records of the patients. Data on the diagnoses, hospitalization status, and whether erythrocyte transfusion (ERT) was required were also obtained from the medical records. Patient data including age of menarche, characteristics of menstrual cycles (duration, regularity, daily number of pads used), medication history, presence of galactorrhea, systemic symptoms, personal and family history of coagulopathy, and sexual activity were documented. Additionally, initial laboratory test results encompassing complete blood count, peripheral blood smear analysis, ferritin levels, prothrombin time (PT), and activated partial thromboplastin time (aPTT) were recorded for analysis (7).
Patients with HMB since menarche, bleeding after surgical and dental procedures, frequent epistaxis or gingival bleeding, and a family history of bleeding disorders were evaluated by a hematologist for possible coagulopathy. Patients suspected of having coagulopathy underwent additional evaluation including Von Willebrand factor (VWF) assay, factor VIII assay, and ristocetin cofactor tests to further assess their coagulation status (8). In patients with active bleeding, human chorionic gonadotropin (hCG) test and pelvic ultrasound (USG) were performed when pregnancy was suspected. Pelvic imaging was not routinely performed due to the retrospective nature of the study and lack of clinical indications in certain cases. Patients were evaluated in terms of obesity according to Body Mass Index (BMI).
In AUB, frequent (intervals of less than 21 days), infrequent (intervals of more than 45 days), prolonged (lasting longer than seven days), or heavy (blood loss of over 80 mL) menstrual cycles may be present. Heavy bleeding has been defined as menstruation that interferes with daily activities in which more than six pads per day are used for longer than seven days (1).
In the presence of anovulation, menstruation that occurs more frequently than every 21 days or is excessive, and serum progesterone levels less than 0.5 ng/mL at the time of diagnosis, known causes of AUB have been excluded. Patients were also classified according to hemoglobin levels. A hemoglobin level of 12 g/dL and above was classified as mild bleeding, 10 - 12 g/dL as moderate, 8 - 10 g/dL as heavy, and a hemoglobin level below 8 g/dL was classified as severe bleeding (19). Patients were divided into two groups: Group 1, consisting of patients with hemoglobin levels below 10 g/dL, and group 2, consisting of patients with hemoglobin levels above 10 g/dL. The two groups were compared in terms of clinical and laboratory characteristics on admission.
All patients with severe anemia and patients with hemoglobin levels below 10 g/dL and active bleeding were hospitalized. Among the patients with hemoglobin levels above 10 g/dL, those who had active heavy bleeding and those who had dizziness and tachycardia were hospitalized. For the remaining patients, treatment and follow-up in the outpatient clinic was planned.
In all patients with anemia (hemoglobin levels < 12 g/dL), oral elemental iron treatment of 60 - 120 mg/day was initiated. Patients with hemoglobin levels above 12 g/dL and no active heavy bleeding were monitored without treatment. Oral iron plus combined oral contraceptives (COC) or oral iron plus NSAIDs (ibuprofen and naproxen) were administered to patients with hemoglobin levels of 10 - 12 g/dL and active bleeding, depending on the intensity of bleeding. Tablets containing 3 mg drospirenone and 0.03 mg ethinylestradiol were administered to patients with active bleeding and severe anemia (Hb < 10 g/dL). Three to four tablets of COCs per day were administered until menstrual bleeding stopped. After the bleeding stopped, doses of COCs were reduced by one tablet every two days, and eventually, treatment was continued with a single dose per day. Treatment with one tablet per day was continued until the hemoglobin levels increased to above 10 g/dL. After the hemoglobin levels increased to above 10 g/dL, treatment with COC was continued cyclically for 3 - 6 months. Antiemetic therapy was administered to patients receiving COC treatment of more than two tablets per day when necessary. Patients who received COCs were monitored for deep vein thrombosis and other complications for one year after the end of treatment. Patients who were thought to have contraindications to estrogen therapy received progestin-only hormone therapy (10 mg/day medroxyprogesterone). Erythrocyte suspension transfusion was performed in adolescents with active heavy bleeding and hemodynamic instability.
The single-center nature of the study limits generalizability. Additionally, the retrospective design may introduce selection bias.
3.2. Statistical Methods
Data were entered into the computer and analyzed using SPSS software, version 25.0. The results were presented as frequency, percentage, mean, standard deviation (SD), median, and interquartile range (IQR). The normality of continuous variables was evaluated using the Kolmogorov-Smirnov test. The independent samples t-test was used to compare anemia status with age, height, weight, age of menarche, and follicle-stimulating hormone (FSH) levels. The Mann-Whitney U test was used to compare other continuous variables, and the chi-square test (or Fisher’s exact test when needed) was used for the comparison of categorical variables. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Participants and Descriptive Data
In the study, 167 adolescent female patients with AUB were included. The mean age of the participants was 13.70 ± 1.74 years, and the mean age of menarche was 11.94 ± 0.99 years. In 41.3% (n = 69) of the patients, hemoglobin levels were above 12 g/dL; in 22.8% (n = 38) of the patients, hemoglobin levels were 10 - 12 g/dL, and in 35.9% (n = 60) of the patients, hemoglobin levels were below 10 g/dL.
In group 1, 35.8% of the patients had hemoglobin levels below 10 g/dL, while in group 2, 64.1% of the patients had hemoglobin levels above 10 g/dL. Out of the 60 patients in group 1, 54 required hospitalization, with 30 of these patients receiving erythrocyte transfusion. Erythrocyte transfusion was not performed in any of the patients in group 2.
In terms of etiological factors, anovulation was present in 84.4% (n = 141) of the patients, and PCOS was present in 11.4% (n = 19) of the patients. The study revealed that among the patients assessed, two individuals were diagnosed with VWD, another two were found to have hypothyroidism, two patients had structural anomalies, and one patient was identified with thrombocytopenia. None of the patients were diagnosed with pregnancy or hyperprolactinemia.
The findings indicated that 11.4% (n = 19) of the patients were classified as overweight or obese, 24% (n = 40) were categorized as underweight, while the majority, 64.7%, were within the normal weight range. Two of the 19 overweight and obese patients were diagnosed with PCOS, and 17 were diagnosed with AUB due to anovulation. Nine of these patients were hospitalized, and ERT was administered to five of them.
The two most common treatments were COCs plus iron (45.5%) and iron alone (28.7%), respectively. Medroxyprogesterone acetate and iron treatments were initiated in five patients with a family history of thromboembolism. Erythrocyte transfusion was performed in 18% (n = 30) of the patients (Table 1). Among the patients who were hospitalized, 55.5% (30 out of 54) required ERT. Additionally, all patients who received ERT had hemoglobin levels below 8 g/dL upon admission.
| Variables | No. (%) |
|---|---|
| Hb level (g/dL) | |
| Severe (< 8) | 36 (21.5) |
| Heavy (8 - 10) | 24 (14.3) |
| Moderate (10 - 12) | 38 (22.7) |
| Mild (> 12) | 69 (41.3) |
| Etiology | |
| Anovulation | 141 (84.4) |
| PCOS | 19 (11.4) |
| VWD | 2 (1.2) |
| Hypothyroidism | 2 (1.2) |
| Thrombocytopenia | 1 (0.6) |
| Structural anomaly | 2 (1.2) |
| Treatment | |
| COCs + Iron | 76 (45.5) |
| Iron | 48 (28.7) |
| No treatment | 26 (15.5) |
| Iron + NSAID | 12 (7.2) |
| Progesterone + Iron | 5 (3) |
| ERT tx | |
| No | 137 (82) |
| Yes | 30 (18) |
Abbreviations: Hb, hemoglobin; PCOS, polycystic ovary syndrome; VWD, Von Willebrand disease; COCs, combined oral contraceptives; ERT, erythrocyte transfusion.
4.2. Outcome Data
The mean age of group 1 (14.26 ± 1.86 years) was higher compared to group 2 (13.39 ± 1.60 years) (t = 3.19; P = 0.002). The weight (z = 2.16; P = 0.032), BMI (z = 3.53; P = 0.000), menstrual period duration (z = 3.24; P = 0.001), pulse rate (z = 8.90; P = 0.000), and number of pads used per day (z = 43.50; P = 0.000) were higher in group 1 than in group 2, while systolic (z = 5.67; P = 0.000) and diastolic blood pressure (z = 4.47; P = 0.000), hemoglobin (Hb) levels on admission (z = 10.71; P = 0.000) and ferritin levels on admission (z = 89.47; P = 0.000) were lower in group 2. The rate of hospitalization in group 1 (86.4%) was significantly higher than that of group 2 (2.8%) (χ = 120.4; P = 0.000). No statistical difference was observed between the two groups in terms of other variables (P > 0.05). Comparison of clinical and laboratory variables between group 1 and group 2 are presented in Table 2.
| Variables | Hb < 10 g/dL | Hb ≥ 10 g/dL | Test Statistic | P-Value |
|---|---|---|---|---|
| Age (y) | 14.26 ± 1.86 | 13.39 ± 1.60 | 3.19 | 0.002 b, c |
| Height (cm) | 156.10 ± 6.06 | 155.79 ± 6.45 | 0.31 | 0.760 b |
| Height SDS | -0.46 ± 0.99 | -0.17 ± 1.02 | 1.81 | 0.072 b |
| Weight (kg) | 51.43 ± 9.56 | 48.15 ± 9.36 | 2.16 | 0.032 b, c |
| Weight SDS | -0.22 ± 1.34 | -0.33 ± 1.22 | 0.51 | 0.608 b |
| BMI (kg/m2) (mdn; IQR) | 20.85; 2.67 | 18.80; 3.20 | 3.53 | 0.000 c |
| BMI SDS | 0.04 ± 1.21 | -0.23 ± 1.07 | 1.54 | 0.126 b |
| Menstruation age (y) | 11.99 ± 1.07 | 11.91 ± 0.95 | 0.54 | 0.589 b |
| Menstrual period (y) (mdn; IQR) | 1.75; 2.25 | 1.10; 1.50 | 3.24 | 0.001 c |
| Pulse (min) (mdn; IQR) | 102.00; 20.00 | 80.00; 8.00 | 8.90 | 0.000 c |
| Systolic blood pressure (mmHg) (mdn; IQR) | 90.00; 15.00 | 100.00; 5.00 | 5.67 | 0.000 c |
| Diastolic blood pressure (mmHg) (mdn; IQR) | 60.00; 10.00 | 60.00; 0.00 | 4.47 | 0.000 c |
| Hb level (g/dL) (mdn; IQR) | 7.60; 2.05 | 12.50; 1.80 | 10.71 | 0.000 c |
| Ferritin (ng/mL) (mdn; IQR) | 3.00; 3.00 | 16.00; 18.00 | 9.47 | 0.000 c |
| Progesterone (ng/mL) (mdn; IQR) | 0.12; 0.17 | 0.18; 0.25 | 0.25 | 0.806 |
| FSH (IU/L) (mdn; IQR) | 4.92 ± 1.74 | 5.31 ± 1.84 | 1.33 | 0.185 b |
| LH (IU/L) (mdn; IQR) | 5.23; 8.91 | 5.98; 6.50 | 0.20 | 0.844 |
| E2 (pg/mL) (mdn; IQR) | 47.86; 33.09 | 53.00; 37.76 | - | 0.22 |
| Number of pads per day (mdn; IQR) | 5.00; 2.00 | 4.00; 2.00 | 3.50 | 0.000 c |
| Family history of AUB | 0.08 | 0.775 d | ||
| No | 52 (86.7) | 91 (85.0) | ||
| Yes | 8 (13.3) | 16 (15.0) | ||
| Hospitalization | 120.4 | 0.000 c, d | ||
| No | 8 (13.6) | 103 (97.2) | ||
| Yes | 51 (86.4) | 3 (2.8) |
Abbreviations: mdn, median; IQR, interquartile range; Hb, hemoglobin; FSH, follicle stimulating hormone; LH, luteinizing hormone; E2, estradiol; SDS, standard deviation score; BMI, Body Mass Index; SD, standard deviation; BP, blood pressure.
a Values are expressed as No. (%) or mean ± SD, unless otherwise indicated.
b The independent samples t-test.
c A P-value of < 0.05 is considered statistically significant.
d Chi-square, Mann-Whitney U test was used in other comparisons.
5. Discussion
Our findings suggest that 35.8% of AUB cases presented with hemoglobin levels below 10 g/dL. Among these cases, approximately 32.2% required hospitalization, with nearly one in every five patients undergoing erythrocyte suspension transfusion. In line with existing literature, anovulation emerged as the predominant cause, accounting for 84.4% of cases (4, 5, 20). Other identified causes included PCOS, VWD, uterine structural anomalies, hypothyroidism, and thrombocytopenia. Previous studies focusing on the etiology of AUB in hospitalized adolescent patients reported coagulation disorder incidences ranging from 5 - 28% (13, 21). Specifically, a review of 988 female cases with HMB revealed a VWD rate of 13% (22). In our study, the prevalence of coagulation disorders was lower at 1.8%, likely due to the exclusion of patients with known cancer or hematological disease. However, despite its low occurrence, coagulation disorders accounted for 10% of cases requiring ERT, underscoring the necessity of evaluating all adolescent patients presenting with severe AUB for bleeding diathesis.
Untreated AUB can lead to severe anemia, hemodynamic instability, and even heart failure if not promptly addressed. Previous studies have reported ERT rates ranging from 22.7% to 22.8% among AUB cases, with a significant increase observed (35.7 - 41.8%) in patients presenting with hemoglobin levels below 10 g/dL (7, 20). In our study, ERT was administered in 18% of all cases, with a higher rate of 50% among those with hemoglobin levels below 10 g/dL. Notably, 86.4% of patients with hemoglobin levels below 10 g/dL required hospitalization, indicating the severity of their condition. These patients exhibited lower systolic and diastolic blood pressures and higher daily pad usage compared to those with higher hemoglobin levels. This underscores the importance of recognizing AUB before hemoglobin levels drop below 10 g/dL.
Despite similar ages of menarche between groups with hemoglobin levels below and above 10 g/dL, patients with higher hemoglobin levels were admitted to the hospital earlier. Therefore, routine inquiry into adolescent menstrual patterns during pediatric outpatient clinic visits is crucial, as some adolescents may be unaware of their abnormal bleeding patterns, given the common irregularity of menstrual cycles during adolescence. Collaborative efforts between health and education authorities are warranted to enhance healthcare professionals’ and families’ awareness and adolescents' knowledge regarding menstruation.
The primary objectives of HMB treatment include ensuring hemodynamic stability, correcting anemia, regulating menstrual cycles, and improving quality of life. First-line treatment typically involves COCs (19, 23). Our study demonstrated that COCs with iron (45.5%) were the most frequently utilized treatment, resulting in cessation of bleeding within two days in COC users. No serious side effects were observed, except for nausea associated with COC treatment, particularly among those using two or more tablets daily. Importantly, no thromboembolic events were observed during the one-year follow-up period following COC use. Progestins represent an alternative treatment for AUB, particularly in adolescents with contraindications to estrogen use (19). In our study, medroxyprogesterone acetate (10 mg/day) was administered in 3% of patients with HMB due to a family history of thromboembolism, achieving bleeding control within three days of treatment initiation. While genetic testing to determine thrombosis tendency was not conducted in our study, evidence suggests that routine genetic testing for COC use is unnecessary (20), particularly considering the low risk of thrombosis associated with COC use in patients with homozygous mutations in the MTHFR gene.
Abnormal uterine bleeding significantly impacts quality of life, leading to decreased school attendance and limited participation in sports and social activities. While our study confirmed the adverse effects of AUB on quality of life, the absence of a standardized quality of life scale for evaluation represents a limitation. The single-center nature of the study limits generalizability. Additionally, the retrospective design may introduce selection bias. This study demonstrates that anovulation is the leading cause of AUB in adolescents, consistent with previous literature. Patients with severe anemia (hemoglobin < 10 g/dL) require hospitalization and may need transfusion.
The retrospective design and single-center setting limit the generalizability of our findings. Routine use of pelvic imaging and collection of long-term follow-up data are recommended areas for future research. In conclusion, anovulation and PCOS are the primary causes of AUB in adolescents. A thorough evaluation, including screening for bleeding disorders, is essential, particularly in patients with severe anemia. Educating families and improving awareness can help prevent delayed diagnosis and severe complications. A wide differential diagnosis should be considered when evaluating adolescents with AUB.