1. Background
Premature births pose significant global health problems (1, 2). There are an estimated 119 million premature live births worldwide (3). The proportion of preterm and term babies born in Asia is higher compared to the rest of the world (1, 2, 4). Babies born prematurely are vulnerable to serious infections and feeding problems (5).
Birth weight is one of the most important indicators of infant health, especially in preterm babies, and is considered the best way to measure the outcome of a pregnancy. It is also the most important factor in determining the incidence of diseases or death in infants. The World Health Organization (WHO) reports the growth of children every year based on variables such as weight, height, and head circumference, with a particular emphasis on children’s weight (6).
In Iran, the process of child growth monitoring for those under 6 years old has been integrated into the healthcare system. Many efforts have been made in this regard, including providing sufficient equipment, printing growth charts, and training staff (7).
Modified Fenton growth curves are based on an international cohort study of 4 million infants. Olsen charts include more than 250,000 infants born in the United States between 22 and 41 weeks of gestation, and along with WHO growth charts, they can be used to monitor postnatal growth up to the 50th week after pregnancy. The number of infants born at 22 - 24 weeks in both charts is very low (less than 1% of the total cohort study), thus limiting the generalization of these charts (8). Recently, growth charts have been published by the Vermont Oxford Network (VON), estimating the birth rate of 156,000 infants in the United States. Vermont Oxford Network charts include more preterm infants (9). The growth pattern of preterm infants is very diverse, reflecting the evolution of infant care and the prolonged survival of infants with lower gestational ages (10). Growth is not constant during fetal, infant, and childhood periods. Some parameters include a growth rate of 15 - 30 grams per day and a height increase of one centimeter per week, but these are only suitable references for a limited time (11). Growth monitoring in infants is approved as a criterion for evaluating a good state of health (12).
2. Objectives
Preterm and low birth weight infants are at a higher risk for developmental disorders. Therefore, this group requires very accurate and regular growth monitoring, which should be done with a thorough understanding of their previous history, events, and current problems to accurately anticipate their growth patterns (13). Consequently, we decided to determine the frequency of postpartum growth retardation in preterm infants through a longitudinal follow-up study.
3. Methods
3.1. Study Design and Participants
This longitudinal follow-up study was conducted at the Neonatal Intensive Care Unit, Pediatric Department, Afzalipour Medical Education and Health Center, Kerman University of Medical Sciences, from February 2020 to February 2021. We included 286 preterm singleton infants weighing less than 2500 g and with a gestational age of less than 37 weeks. Thirty-one infants were excluded due to a lack of regular visits.
These infants were examined at birth by a NICU physician, and their weight was measured with a Seca Scale and recorded in grams. The scale was calibrated at the beginning of each day and as needed. The head circumference of the infants was measured with a flexible tape and recorded in centimeters. The procedure for measuring height in the clinic involved placing the infant supine on a table, holding the head fixed, extending the heel of the right foot, and measuring with a millimeter ruler, with the measurement recorded in centimeters.
Parents' information and the growth pattern of the infants, including anthropometric characteristics such as birth weight, height, and head circumference at birth, and at ages 7, 14, and 28 days, and then monthly up to 6 months, were recorded in a special questionnaire. The Z-scores for these ages were calculated based on the infants' uncorrected gestational age. The Fenton chart was used to calculate the Z scores for infants up to 50 weeks of gestational age, and thereafter up to 6 months based on the standard WHO growth chart.
Infants with severe congenital and chromosomal abnormalities, twins or multiples, those lacking regular visits for growth monitoring, and infants with smoking mothers were excluded from the study.
3.2. Data Collection
Data collection for the questionnaire consisted of four parts:
Part 1: Information about the demographic characteristics of the mother and her husband was collected, including the mother's age, family size, education levels of both parents, employment status, and monthly family income. Part 2: Infants' anthropometric parameters, including height, weight, and head circumference, were plotted on the Fenton preterm growth chart at birth, at the end of the first week, at ages 14 and 28 days, and then monthly up to 6 months. Part 3: This section recorded the infant's calorie requirements based on weight, gestational age, and any diseases affecting nutritional status. It also determined the type of diseases impacting the infant's nutrition. Part 4: This part assessed the type of feeding during hospitalization (parenteral or enteral, gavage or oral) and the type of milk consumed, including breast milk, infant formula, and milk from a milk bank. It also recorded complementary feeding (fortifiers, vitamins, iron) during the first six months after birth.
Factors affecting infant feeding during hospitalization were evaluated by the infant's therapist. These factors included: (A) nutritional content, especially total parenteral nutrition (TPN); (B) practical questions about enteral nutrition for preterm infants; (C) gastrointestinal surgical issues [gastroscopy, necrotizing enterocolitis (NEC), esophageal atresia]; (D) factors limiting the transition from gavage feeding to oral feeding.
The results obtained during hospitalization and follow-up periods in the clinic were compared between two groups. Infants with birth weights between the 10th and 90th percentiles were classified as appropriate for gestational age (AGA), while those below the 10th percentile were considered small for gestational age (SGA).
3.3. Statistical Data Analysis
Data were coded and analyzed using statistical package for the social sciences (SPSS) software version 20. To describe the data, the mean and standard deviation were used for quantitative variables, while frequency and percentage were used for qualitative variables. The chi-square test was employed to investigate the relationship between different variables, such as birth weight and nutritional status, with postnatal growth failure (PGF) across different follow-up intervals.
3.4. Ethics Approval and Consent to Participate
The study was designed with consideration of ethical aspects and was conducted according to the Declaration of Helsinki 1975, as revised in 2008. It was approved by the Institutional Review Board and Medical Ethics Committee of Kerman University of Medical Sciences (IR.KMU.AH.REC.1399.059). The study was explained in detail to the parents or legal guardians of the participant children, and written informed consent was obtained from them.
4. Results
In the present study, 255 infants were included, comprising 140 boys (54.9%) and 115 girls (45.1%) (Table 1).
| Variables | Values a |
|---|---|
| Gender | |
| Male | 140 (54.9) |
| Female | 115 (45.1) |
| Birth weight | |
| AGA | 205 (80.4) |
| SGA | 50 (19.6) |
| Route of delivery | |
| C/S | 186 (72.9) |
| NVD | 69 (27.1) |
| Gestational age (all) (week) | 33.39 ± 2.25 |
| < 34 | 31.3 ± 1.4 |
| ≥ 34 | 35.02 ± 0.79 |
| Gestational age at discharge (week) | |
| AGA | 34.8 ± 1.9 |
| SGA | 36.6 ± 2.4 |
| Birth weight (all) (week) | 1945.9 ± 433.7 |
| < 34 | 1656.1 ± 395.7 |
| ≥ 34 | 2184.0 ± 297.8 |
| Birth head circumference (cm) | 30.9 ± 2.04 |
| Birth height (cm) | 45.4 ± 3.8 |
| APGAR score (AGA&SGA) | 8.18 ± 1.6 |
| APGAR score AGA | 8.09 ± 1.17 |
| APGAR score SGA | 8.58 ± 1.05 |
Abbreviations: AGA, appropriate for gestational age; SGA, small for gestational age.
a Values are expressed as No. (%) or mean ± SD.
Their growth indices, including weight, height, and head circumference, were measured at birth, at the time of discharge, and monthly thereafter. Postnatal growth failure was defined based on Z-scores: A weight Z-score of less than -1.28 on the Fenton chart, corresponding to less than the 10th percentile. This definition aligns with the Vermont Oxford Network, which evaluates the postnatal growth of preterm infants. At 6 months of age, according to WHO Z-score charts, a score of less than -1.68, which is below the 5th percentile, was considered indicative of growth retardation.
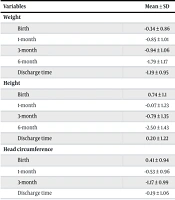
Table 2 and Table 3 display the mean Z-scores for weight, height, and head circumference at birth, discharge, and one, three, and six months. Weight Z-scores ranged from -1.79 to 0.74, height Z-scores ranged from -2.5 to 0.74, and head circumference Z-scores ranged from -1.17 to 0.41. The frequency of deviation from PGF at the time of discharge was 109 infants (42.7%), at one month 79 (31%), at three months 82 (32.2%), and at six months 134 (52.5%).
Among infants with growth retardation at the time of discharge, 70 infants (64.2%) had growth retardation at one month, 60 infants (55%) at three months, and 68 infants (62.4%) at six months. Additionally, 80.4% of infants were classified as AGA, while 19.6% were classified as SGA. Growth retardation increased significantly among SGA infants (P = 0.001).
| Deviation From the Growth Curve at Different Times | No. (%) |
|---|---|
| 1-month-old | |
| z ≤ -1.28 | 79 (31) |
| z > -1.28 | 176 (69) |
| 3-month-old | |
| z ≤ -1.28 | 82 (32.2) |
| z > -1.28 | 173 (67.8) |
| 6-month-old | |
| z ≤ -1.28 | 134 (52.5) |
| z > -1.28 | 121 (47.5) |
| Discharge time | |
| z ≤ -1.28 | 109 (42.7) |
| z > -1.28 | 146 (57.3) |
| Variables | Mean ± SD |
|---|---|
| Weight | |
| Birth | -0.34 ± 0.86 |
| 1-month | -0.85 ± 1.01 |
| 3-month | -0.94 ± 1.06 |
| 6-month | -1.79 ± 1.17 |
| Discharge time | -1.19 ± 0.95 |
| Height | |
| Birth | 0.74 ± 1.1 |
| 1-month | -0.07 ± 1.23 |
| 3-month | -0.79 ± 1.35 |
| 6-month | -2.50 ± 1.43 |
| Discharge time | 0.20 ± 1.22 |
| Head circumference | |
| Birth | 0.41 ± 0.94 |
| 1-month | -0.53 ± 0.96 |
| 3-month | -1.17 ± 0.99 |
| Discharge time | -0.19 ± 1.06 |
In the PGF group, the average time to reach full enteral feeding (100 cc/kg/day of milk) was 11.62 days compared to 6.87 days in the non-PGF group. Growth retardation was more common in infants who took longer to reach full feeding (P = 0.001). Growth retardation at discharge was significantly inversely correlated with the duration of mechanical ventilation, with 7 days being the median duration (P = 0.001).
There was a significant positive correlation between oxygen duration and growth retardation at discharge in the PGF group compared to the non-PGF group (P = 0.001). The mean NPO duration was 3.56 days and correlated significantly with growth retardation at discharge (P = 0.004). Among preterm infants, 35% experienced various complications, and there was a significant relationship between PGF at discharge and hospitalization problems (P = 0.001). Infants who spent longer in the hospital had higher growth retardation at discharge (P = 0.001). Total parenteral nutrition duration directly correlated with PGF at discharge (P = 0.001), and low birth weight significantly correlated with PGF at discharge (P = 0.001) (Table 4).
| Variables | z ≤ -1.28 | z > -1.28 | P-Value |
|---|---|---|---|
| Birth weight | 0.001 | ||
| AGA | 65 (31.7) | 140 (68.3) | |
| SGA | 44 (88) | 6 (12) | |
| NPO | 0.04 | ||
| Yes | 85 (78) | 99 (68.3) | |
| No | 24 (22) | 46 (31.7) | |
| Hospitalization causes | 0.365 | ||
| CHD | 2 (1.8) | 1 (0.7) | |
| Icter | 16 (14.7) | 38 (27.1) | |
| Respiratory distress | 70 (64.2) | 80 (57.1) | |
| Asphyxia | 5 (4.6) | 9 (6.4) | |
| Early Sepsis | 0 (0) | 1 (0.7) | |
| Gastrointestinal bleeding | 0 (0) | 1 (0.7) | |
| Seizure | 2 (1.8) | 1 (0.7) | |
| Intestinal obstruction | 7 (6.4) | 6 (3.4) | |
| TEF | 3 (2.8) | 1 (0.7) | |
| Renal anomaly | 0 (0) | 1 (0.7) | |
| Prematurity | 4 (3.7) | 1 (0.7) | |
| Clinical problems during hospitalization | 0.001 | ||
| Yes | 48 (44.4) | 25 (17.2) | |
| No | 60 (55.6) | 120 (82.8) | |
| Route of delivery | 0.08 | ||
| Vaginal | 29 (31.9) | 40 (35.7) | |
| Caesarean section | 62 (68.1) | 72 (64.3) | |
| Type of feeding | 0.081 | ||
| Breast milk | 96 (71.6) | 101 (83.5) | |
| Formula | 13 (9.7) | 7 (5.8) | |
| Breast milk and formula | 25 (18.7) | 13 (10.7) | |
| Type of consuming supplementation and vitamins | 0.969 | ||
| Incomplete consumption of supplementation and vitamins | 18 (22.5) | 35 (20.8) | |
| Full consumption of supplementation and vitamins | 62 (77.5) | 133 (79.2) | |
| Weight of birth (g) | 1679.9 ± 446.6 | 2048.61 ± 383.11 | 0.001 |
| APGAR score | 8.04 ± 1.2 | 8.29 ± 1.09 | 0.09 |
| Gestational age at discharge time (week) | 35.58 ± 1.9 | 34.3 ± 1.6 | 0.001 |
| Duration of full enteral feeding (day) | 11.6 ± 8.7 | 6.8 ± 5.8 | 0.001 |
| Duration of oxygen requirement (day) | 11.9 ± 8.0 | 7.9 ± 5.5 | 0.001 |
| Duration of mechanical ventilation (day) | 7.0 ± 5.6 | 4.8 ± 3.8 | 0.007 |
| Length of hospitalization (day) | 15.94 ± 11.99 | 8.61 ± 6.45 | 0.001 |
| Duration of receiving amino acid (day) | 13.4 ± 8.6 | 8.6 ± 6.3 | 0.001 |
Abbreviations: CHD, congenital heart disease; TEF, tracheoesophageal fistula.
a Values are expressed as No. (%) or mean ± SD.
5. Discussion
Hospitalized premature and low birth weight infants are particularly vulnerable to developmental disorders. This study showed that surgical problems, feeding intolerance, NEC, and other factors were significantly inversely related to normal postnatal growth in this population.
In this study, there was no significant relationship between growth retardation at the time of discharge and gestational age. A study conducted by Fenton and Kim in 2013 at six major health centers in Germany, Italy, the United States, Australia, and Scotland indicated that although the growth pattern of preterm infants was generally consistent with intrauterine growth, the greatest deviation in weight gain among preterm infants, fetuses, and term infants occurred between 37 and 40 weeks of age. The difference in our study may be due to it being a single-center study with a smaller sample size, and the growth stages of infants were not examined and compared separately in each gestational age group (14).
In this study, there was a direct relationship between PGF at discharge and the length of hospital stay. In a study by Liao et al. in 2019 on 2124 VLBW infants, the prevalence of growth retardation at 6 months post-discharge was 17.3%, and infants with longer hospital stays had poorer growth outcomes. Necrotizing enterocolitis leading to surgery and RDS treated with surfactant also negatively affected growth, consistent with our findings (15).
Our study found that infants in the SGA group had more growth retardation at discharge than those in the AGA group. A study conducted by Huh et al. in Korea in 2017 on 129 SGA infants showed no significant difference between SGA and AGA groups in postnatal growth up to 24 months of age, except for height at 6 months. Low gestational age and low birth weight were identified as risk factors for growth deficits at 6 months of age when comparing catch-up growth in AGA and SGA infants (16).
In our study, hospitalization problems such as NEC, pneumothorax, sepsis, and intraventricular hemorrhage (IVH) were generally associated with post-discharge growth failure. A study by Lee et al. in 2017 showed that post-discharge growth failure was higher in infants with comorbidities such as RDS, BPD, PDA, NEC, IVH, ROP, PVL, and sepsis in the PGF group (17).
In the present study, there was no significant relationship between the Apgar score at 1 and 5 minutes and post-discharge growth failure. The study by Park et al. concluded that the Apgar score at 5 minutes in the PGF group was significantly lower than in the non-PGF group. Additionally, in this study, the duration of mechanical ventilation, length of hospital stay, duration of TPN, and the time needed to reach full enteral nutrition (100 cc/kg/day) in the PGF group were longer than in the non-PGF group, which is consistent with our findings (18).
In the present study, growth failure after discharge was 42.7%, which decreased to 31% and 32.2% at one month and three months, respectively. However, by the age of six months, the rate had risen to 52.5%. The study conducted by Horbar et al. showed that infants gained 12 grams per kilogram of body weight per day after birth. Additionally, during hospitalization, the rate of growth failure decreased from 64.5% to 50.3%, and the rate of severe growth failure decreased from 39.8% to 27.5%. In our study, the increase in growth failure at 6 months of age could be attributed to the comparison of preterm infants with WHO term infants’ charts, which are not the standard growth pattern for preterm infants. By the modified age of two years, these infants generally regain weight in accordance with infants of the same age. In Iran, there are still no standard reference charts to assess the growth of these children from the age of 50 weeks postnatal until they reach the modified age of two years (13).
In the present study, growth failure at the time of discharge was significantly higher in the SGA group. In the study by Park et al. (18) they concluded that infants at a post-conception age (PCA) of 40 weeks had 58% and 55% growth failure in height and weight, respectively. Additionally, at PCA 24 months, in terms of height and weight, they had 24% and 18% growth failure, respectively. The rate of growth failure in SGA infants was higher than in infants with appropriate gestational age at PCA 24 months, which is consistent with our study.
In this study, the duration of TPN and the duration of neonatal NEC were significantly directly related to PGF at the time of discharge. Additionally, growth failure at discharge was significantly higher in infants who took longer to reach full feeding. The study conducted by Ehrenkranz et al. in 1999 showed that the weight gain of the infants studied was approximately 4.14 - 16 g per day, which is similar to the rate of intrauterine growth. However, most infants with a gestational age of 24 - 29 weeks who were discharged from the hospital did not reach the mean fetal weight reference at postmenstrual gestational age. Infants AGA without chronic lung disease, IVH, NEC, and late-onset sepsis gained more weight than those with these complications. Additionally, rapid weight gain was associated with shorter TPN duration. Furthermore, providing an earlier start of 75% of the daily fluid intake in the form of enteral nutrition and achieving full enteral feeding more quickly was associated with faster weight gain, which is consistent with our findings (19).
In another study conducted by Aramesh et al. in 2009 in Ahvaz, they concluded that although the mean growth rate of infants with low birth weight is very close to the mean growth rate of infants with normal birth weight, the growth of this group of infants is still not normal at 6 months of age. This finding is similar to our study at 6 months of age (20).
In another study conducted by Rashidi et al. in Mashhad in 2018, they concluded that there is a difference between the growth charts for Iranian infants according to their age and gender compared to the Fenton growth charts. This may be due to factors such as ethnicity, region, socio-economic factors, maternal and fetal health, and nutritional status during pregnancy, which were also observed in the present study. However, due to our small sample size, we cannot definitively judge this issue (21).
This is the first longitudinal follow-up study in Iran that evaluated the postnatal growth of premature babies admitted to the hospital and also followed their growth after discharge. One limitation of this study is that it was conducted at a single center, and further studies are required to generalize the findings. The development of specific growth charts for premature babies in developing and low-income countries will be possible if several comprehensive studies are conducted in other cities. Additionally, there is a lack of standard and appropriate TPN solutions for premature and low birth weight babies, tailored to their physiological and metabolic conditions. This study did not address this issue, which is a significant concern in low-income countries.
5.1. Conclusions
The population of premature babies, many of whom did not survive in the past, now constitutes a significant part of the birth rate. However, there is no general agreement on growth curves for preterm infants, even in developed countries. Regular growth monitoring of preterm infants is crucial for the timely detection and prevention of growth disorders, allowing them to follow a normal developmental trajectory and enabling families to have confidence in their progress without unnecessary interventions or stigma. In Iran, the increase in growth failure at 6 months of age could be due to preterm infants being compared to WHO term infant charts, which do not represent their standard growth pattern. Iran lacks standardized charts to assess preterm infant growth up to 2 years, and also lacks access to specialized TPN solutions suitable for their physiological needs. Addressing these gaps through national initiatives, aggressive enteral feeding, and the use of colostrum is imperative to support the wellbeing of preterm infants in the country.