1. Background
Rectal prolapse is prevalent in children between the ages of one and four. The overwhelming majority of cases of pediatric rectal prolapse are idiopathic, with no known cause. However, anatomical and viral factors contribute to the development of this disease (1). Rectal prolapse is initially treated conservatively for at least six months, and if there is no response and symptoms persist, surgical intervention is required (2). Some systemic disorders, nerve damage, and defects, such as cauda equina syndrome, can cause rectal prolapse (3, 4). Therefore, it is important to consider and treat these as well. Background conditions such as constipation and diarrhea can result in prolapse after each defecation, causing anxiety in the child and parents, necessitating frequent emergency visits and hospitalizations, and requiring repetitive manipulation of the rectum to reduce the prolapse (5-7). Therefore, a variety of surgical procedures through the perineum or abdomen have been suggested for the treatment of prolapse that is resistant to medical treatment (8). Proctopexy through the anus with submucosal injection of a sclerosing agent appears to be associated with fewer complications and quicker recovery, and it can be readily repeated to achieve the desired treatment outcome (9). Compared to Deflux, dextrose 50% as a sclerosant is considerably more accessible and inexpensive. Many trials have used the sclerosing medication Deflux, which is highly effective in reducing recurrences and adverse effects, such as the development of fistulas and abscesses (10). In children with rectal prolapse, medical treatment lasts at least three to six months and consists of nutritional style and pattern modification, elimination of the underlying cause, and parental training to reduce the prolapse (7, 11). Surgical or non-surgical interventions are considered if a patient does not respond to medical treatment (12). Most surgeons oppose different surgical techniques, such as intra-abdominal rectal prolapse repair (13). Perianal surgical techniques like Thiersch wire are not commonly used on children (14). In recent years, submucosal and perirectal injections for sclerotherapy have become more prevalent and have been reported to have satisfactory outcomes and fewer adverse effects (15). To avoid damaging the entire anal canal, it is critical to carefully select the type, depth, and volume of sclerosant injected. Sclerotherapy has been linked to rare complications such as infection, mucosal sloughing, perianal fistula, and abscess formation (16). As the advancement of less invasive techniques has resulted in improved safety and efficiency, this study investigates the results and adverse effects of perirectal sclerotherapy administered via the anus in infants with rectal prolapse.
2. Objectives
The objective of this study was to evaluate the results and adverse effects of perirectal sclerotherapy administered via the anus in infants with rectal prolapse. The study aimed to assess the efficacy and safety of this approach as a treatment option for pediatric rectal prolapse.
3. Methods
This study is a non-blinded, randomized clinical trial involving 40 patients with rectal prolapse who did not respond adequately to supportive treatments over three years (2019 - 2021). Patients with resistant rectal prolapse who remained symptomatic after three to six months of conservative treatment were eligible to participate in the study. Patients with underlying diseases (such as anorectal anomaly, myelomeningocele, Hirschsprung disease, and a history of anoplasty) were excluded, as were those older than 15 years, those with rectal polyps or rectal bleeding diagnosed clinically or via colonoscopy, and those with prolapse due to neuropathic causes such as meningocele or acute rectal prolapse.
The patients included in this study initially received three to six months of conservative treatment, which included dietary and toilet training modifications and, if necessary, treatment for constipation. A patient becomes a candidate for perirectal injection of a sclerosant agent if there is no complete response to non-surgical treatment. Complete colon preparation was not required for the patients. They were transferred to the operating room following a single saline enema and a single bowel movement on the morning of surgery. After general anesthesia was administered in the lithotomy position, the anus and rectum were examined using a finger and a speculum. Dextrose 50% was injected 2 - 3 cm above the pectinate line in three locations at 3, 6, and 9 o'clock positions (1 cc/kg at 6 o'clock and 0.5 cc/kg at 3 and 9 o'clock). It is important to note that Dextrose 50% should not be administered at the 12 o'clock position. This precaution is taken due to anatomical considerations, as the passage of the urethra in boys and the vaginal wall in girls may be affected if the injection is given at this particular point.
The formation of mucous swelling in the corresponding quadrants indicates appropriate injection. Intramucosal injection should be avoided to prevent complications such as necrosis, hemorrhage, and stricture. Patients were discharged the following day after managing the injection's initial complications. They were monitored on the seventh day, three months, and six months later for recurrence and complications, with a second injection administered if a recurrence occurred. During the initial 24 hours of hospitalization, early complications such as pain and erythema at the injection site were observed and managed with analgesics.
Following discharge, close monitoring of the injection site was conducted within the first week to check for any signs of cellulitis or infection. If such indications were present, appropriate treatment with anti-inflammatory medications or antibiotics was administered. Additionally, late complications, including anal canal tightness and recurrence, were evaluated after a six-month period. There was no difference in the anesthetic method or medications administered to laparoscopic versus open-surgery patients. Early complications of stricture were checked through anus and rectal examination, and if necessary, finger examination and, in younger children, bougienage with bougie number 8 to 12.
In addition to clinical examination and, if necessary, anoscopy for stenosis, anti-inflammatory agents for cellulitis, and antibiotics for infection, mid-term and late complications were treated with debridement and rinsing in the case of necrosis. Patient demographics, including age, gender, grade of prolapse, number of injections, initial symptoms at the time of the visit (bleeding and discomfort), and a history of underlying diseases, were collected at the outset. After sclerotherapy, results and complications were recorded, along with the occurrence of complications and recurrence symptoms.
The patients' demographic information was analyzed using descriptive statistical methods and presented in appropriate tables and graphs as frequency and mean ± standard deviation. Quantitative variables were compared using the paired t-test, and qualitative variables were compared using the chi-Square test. A P-value of less than 0.05 was considered statistically significant.
4. Results
This clinical trial included 40 rectal prolapse patients admitted to Tabriz Children's Hospital between April 2019 and March 2020. Table 1 presents the demographic characteristics of the patients.
| Variables | N = 40 |
|---|---|
| Age (y) | 5.62 ± 2.98 |
| Gender | |
| Male | 28 (70) |
| Female | 12 (30) |
| Initial symptoms of sclerotherapy (mon) | 6.60 ± 1.73 |
| Pre-surgery treatments | |
| Constipation treatment | 25 |
| Conservative treatment without medication | 15 |
| Prolapse grade | |
| I | 0 |
| II | 0 |
| III | 10 (25) |
| IV | 30 (75) |
| Initial symptoms | |
| Rectal prolapse | 14 (35) |
| Partial rectal prolapse | 14 (35) |
| No symptoms | 12 (30) |
| Prolapse diagnosis method | |
| Clinical examinations | 10 (25) |
| Photos by parents | 30 (75) |
Patient Demographics a
Table 2 displays the average volume of sclerosing agent injections administered to the intervention group patients in each quadrant.
| Variables | Mean ± SD (cc) |
|---|---|
| 3 o'clock | 8.60 ± 1.50 (6 - 12) |
| 6 o'clock | 10.60 ± 1.60 (8 - 14) |
| 9 o'clock | 8.60 ± 1.60 (6 - 12) |
| Total | 27.80 ± 4.80 (20 - 38) |
The Average Volume of Sclerosant Injection
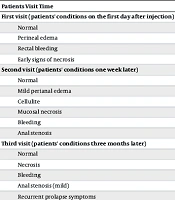
Table 3 presents an evaluation of the health of patients in the intervention group during follow-up visits after the injections.
| Patients Visit Time | Condition; No. (%) |
|---|---|
| First visit (patients' conditions on the first day after injection) | |
| Normal | 36 (90) |
| Perineal edema | 4 (10) |
| Rectal bleeding | 0 (0) |
| Early signs of necrosis | 0 (0) |
| Second visit (patients' conditions one week later) | |
| Normal | 36 (90) |
| Mild perianal edema | 3 (7.5) |
| Cellulite | 1 (2.5) |
| Mucosal necrosis | 0 (0) |
| Bleeding | 0 (0) |
| Anal stenosis | 0 (0) |
| Third visit (patients' conditions three months later) | |
| Normal | 34 (85) |
| Necrosis | 0 (0) |
| Bleeding | 0 (0) |
| Anal stenosis (mild) | 1 (2.5) |
| Recurrent prolapse symptoms | 5 (12.5) |
| Fourth visit (patients' conditions six months later) | |
| Normal | 38 (95) |
| Anal stenosis | 0 (0) |
| Recurrence symptoms–second injection | 2 (5) |
Health Evaluation of Patients During Follow-up Visits Following Injections
As indicated in Table 3, during the three-month follow-up period, signs of relapse were observed in 5 patients, accounting for 12.5% of the total. However, it is noteworthy that 3 of these patients experienced improvement after three months of conservative treatment. At the six-month mark, only two patients (5%) continued to exhibit relapse symptoms, leading to the administration of a second injection. Among these two patients, one individual did not achieve recovery and subsequently underwent surgery.
Table 4 displays the final results of the examined patients compared to the control group. According to the results, the intervention group patients who did not respond to the second injection were subsequently subjected to surgery.
| Variables | No. (%) |
|---|---|
| Complete treatment with the first injection | 34 (85) |
| Partial treatment with the first injection | 4 (20) |
| No treatment with the first injection | 2 (5) |
| Complete treatment in the second injection | 3 (50) |
| No treatment in the second injection | 3 (50) |
The Final Results of the Studied Patients Compared to the Control Group
5. Discussion
In this clinical trial, perirectal sclerotherapy was administered to 40 children with rectal prolapse after supportive treatment. An analysis of age categories revealed that most patients with successful sclerotherapy were between the ages of four and six. According to De La Torre et al. and Mustafa et al., the average age of children with successful treatment for rectal prolapse was also between 4 and 6 years (17, 18).
The majority of our patients (70%) were male. The average duration of symptoms was 22.60 ± 9.73 months. All patients had rectal prolapse of grades III and IV, with partial prolapse being the most prevalent symptom (45%).
In the present study, only one patient had an underlying disease associated with anemia, while the remaining patients had no underlying diseases. At the final grade of rectal prolapse, full-thickness prolapse occurs more frequently in individuals with autism than in the general population (19). Additionally, rather than being a congenital disease, rectal prolapse is associated with children's growth and development, manifesting progressively during the first years of life (20, 21).
According to this study, 90% of patients had a normal state on the first visit (the day following the injection), and only 10% experienced edema at the injection site. At the second examination, conducted a week later, only one patient had cellulitis, and three others had modest anal edema. Three months later, during a visit related to mid-term problems, one instance of mild anal stricture and five cases of prolapse symptoms were noticed again. Two instances of prolapse recurrence were observed during the patient's final visit, related to long-term complications. As a result, 95% of patients in the current study experienced a satisfactory recovery after receiving a sclerosant injection. The therapeutic success rate of sclerotherapy injection in children with rectal prolapse has been found to range between 66% and 100% in prior investigations.
The final examination of our study's patients revealed that 85% of them were completely cured after the initial sclerosant injection, and none experienced a recurrence of their prolapse within six months. However, for the other 15% of patients, partial treatment resulted in a 70% reduction in rectal prolapse after the injection. Sclerotherapy was re-injected in just 5% of cases that did not improve after the first injection. After the second injection, three patients were completely cured, while two continued to experience prolapse symptoms and were referred for surgical intervention.
One of the most successful therapies for rectal prolapse in children is injecting a sclerosing agent into the perirectal space (22). Various sclerosing agents have been used with varying degrees of success. Many different materials can be used for this process, each with advantages and disadvantages (23-26). Among these, hypertonic saline is the most common (27, 28). The sclerosing properties of high-concentration dextrose solutions also make them useful for this purpose (29, 30). The study by Fahmy and Ezzelarab compared the therapeutic effects of several sclerosing drugs, finding that none of the three drugs (95% ethyl alcohol, 5% phenol almond oil, and Deflux) caused serious adverse effects (16). In the current study, dextrose 50% was preferred due to its affordability, availability, and safety. Sclerotherapy with 15% saline has been reported to have a 93.7% success rate by Abeş and Sarihan (31) after the first injection. Similarly, a study conducted by Morrison et al. reported a success rate of 82.4% in sclerotherapy using hypertonic saline (32). Another study conducted by Ezer et al. indicated that phenol almond oil was more effective than cow's milk, 30% saline, 30% dextrose solution, and 70% ethyl alcohol (33). Further investigation indicated that phenol was associated with a 9% incidence of abscesses (16). Based on their findings, Dolejs et al. concluded that sclerotherapy was successful in the vast majority of cases for children older than five years and that subsequent episodes could be halted by providing a second injection in cases of recurrence (34). Additionally, problems like ischiorectal abscess and perianal irritation can be avoided with a submucosal injection (35). Recurrence of prolapse following sclerotherapy is reported to be higher in children who are older and heavier than average (11), which was not investigated in the present study but is recommended for future research on children with rectal prolapse.
The study has several limitations that should be considered when interpreting the findings. Firstly, the sample size is relatively small, consisting of only 40 patients, which may limit the generalizability of the results. A larger sample size would provide more robust and representative findings. Secondly, the study design is non-blinded, which introduces the possibility of bias, particularly in subjective assessments such as symptom improvement. It is important to acknowledge this potential source of bias when evaluating the study's conclusions.
Thirdly, the follow-up period in the study is relatively short, with a maximum duration of six months. The lack of long-term follow-up beyond this period hinders the assessment of treatment efficacy and recurrence rates over a more extended timeframe. Lastly, the study primarily focuses on evaluating the efficacy and safety of perirectal sclerotherapy using a 50% dextrose injection. While this approach is valuable, it is essential to recognize that other relevant factors or treatment modalities may not have been adequately addressed or explored. Considering these limitations will help to ensure a comprehensive understanding of the study's outcomes and their implications.
5.1. Conclusions
This study's findings support the use of 50% dextrose as the primary surgical treatment for rectal prolapse in children younger than 14 years old due to its low cost and ease of implementation. Additionally, sclerotherapy using 50% dextrose has a minimal risk of complications and short recovery times compared to open surgery.