1. Background
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic hepatologic disorder, affecting nearly 38.0% of the global population (1). Non-alcoholic fatty liver disease is characterized by the accumulation of lipids in hepatic cells, known as steatosis (2). If left untreated, NAFLD can progress to Non-alcoholic steatohepatitis (NASH), marked by inflammation and fat infiltration of the liver parenchyma, potentially leading to liver cell apoptosis (3). Non-alcoholic steatohepatitis can then result in fatal conditions such as hepatocellular carcinoma (HCC), hepatic fibrosis, and cirrhosis (4). Despite extensive research on the epidemiology of NAFLD and NASH in adults, data on the pediatric population is limited. However, it is evident that the incidence of NAFLD/NASH among children has been steadily increasing by approximately 1.3% annually, with the highest rates seen in the Middle East (5). This rise in pediatric NAFLD/NASH cases is likely linked to the growing prevalence of childhood obesity and the adoption of a Western diet high in fats and simple carbohydrates, and low in fibers (6).
As NASH is now the second most common reason for liver transplantation in developed countries, it is crucial to prioritize preventative measures, such as early treatment of NAFLD, to address this issue (7). While pharmacological treatments for NAFLD and NASH have not yielded significant results, emphasizing lifestyle modifications and prevention remains key (8). Addressing obesity and improving diet are primary steps in treating and preventing NAFLD/NASH (9).
Various studies have proposed serologic, histologic, and radiologic parameters for diagnosing pediatric NAFLD. Biomarkers such as cytokeratin 18, Fas, Fas ligand, and oxidized low-density lipoprotein have shown elevated levels in NAFLD patients compared to healthy individuals. However, these biomarkers require specialized laboratory equipment that may not be readily available, particularly in resource-limited settings (10). Histological evaluation of liver tissue and assessment of hepatic fibrosis through liver biopsy are crucial for determining mortality risk (11). Alternatively, ultrasound evaluation of the liver parenchyma compared to the right renal parenchyma can provide a highly sensitive and specific diagnosis of NAFLD. In fact, European guidelines recommend ultrasound as the primary diagnostic tool for NAFLD in adults (12, 13). The effectiveness of ultrasound in diagnosing NAFLD in obese children versus normal-weight children requires further investigation.
2. Objectives
This study aims to compare ultrasonographic findings with traditional liver enzyme tests for diagnosing NAFLD in both normal-weight and obese children.
3. Methods
This study followed a case-control protocol and was conducted in Jahrom, southern Iran, on 100 children who attended the Honari clinic of Jahrom University of Medical Sciences. After explaining the course of the study and obtaining written informed consent from the patients’ parents, 50 obese or overweight children and 50 non-obese, age-and sex-matched children underwent ultrasonography and serum biochemical testing to evaluate liver enzymes.
The inclusion criteria of our study were children aged 5 - 18 years with no signs or symptoms of extensive liver failure and no history of consumption of steatogenic compounds. The exclusion criteria were unwillingness of the parents to participate in the study, cirrhosis, or signs and symptoms of this condition including ascites, jaundice, esophageal varices, or splenomegaly. Patients with viral hepatitis, celiac disease, hypothyroidism, Wilson’s disease, autoimmune hepatitis, α1-antitrypsin deficiency, and abetalipoproteinemia were also excluded.
Obesity or overweight in children was defined as a Body Mass Index (BMI) higher than the 85th percentile for their age according to the World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) (14, 15). Weight was measured by a single digital weighing scale with a precision of 0.1 kg, and height was measured by a single stadiometer in a posture where the patients’ shoulders, heels, and buttocks touched the wall and the head kept horizontally with a precision of 0.1 cm.
In addition to the anthropometric evaluation, five milliliters of blood was taken from each participant for the measurement of gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) using an auto-analyzer (BT1500; Biotecnica instrument) and Pars Azmun® standardized kits.
The patients then underwent comprehensive abdominopelvic ultrasonography by a single expert radiologist for the assessment of fatty liver and its grading. Grade I fatty liver was defined as diffusely increased hepatic parenchymal echogenicity with significant periportal and diaphragmatic echogenicity while in grade II fatty liver, periportal echogenicity is obscured. In grade III fatty liver, although the hepatic echogenicity is increased, the diaphragmatic and periportal echogenicity are both obscured (16).
The diagnosis of NAFLD was also made by the use of liver enzymes as suggested by the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) with serum ALT levels above 50 U/L for boys and above 44 U/L for girls as twice the upper limit of normal (17).
The data were entered into IBM SPSS version 24.0. Qualitative variables were reported as frequency and percentages, and quantitative variables were reported as mean and standard deviation. The prevalence of NAFLD was calculated in each group by the defining characteristics of ultrasonography and lab values, and the 95% confidence interval for each prevalence rate was estimated using a one-sample binomial test. The Kappa measure of agreement was used to assess the two diagnostic methods, and Spearman’s correlation test was used to evaluate the correlation of ultrasonographic grading of NAFLD with lab values. Receiver operating characteristics (ROC) curves were drawn for each lab value based on the diagnosis of NAFLD using ultrasonography to find the best cut-off values for these values for the diagnosis of NAFLD. P-values less than 0.05 were considered statistically significant.
This study was conducted in accordance with the Helsinki declaration on research ethics. It was also approved by the Committee of Ethics in Biomedical Research of Jahrom University of Medical Sciences with the code: IR.JUMS.REC.1403.001.
4. Results
A total of 100 participants entered the study, half of whom were overweight or obese. The other half were age-and sex-matched counterparts with a normal BMI. In this study, 58 participants (58.0%) were male and 42 participants (42.0%) were female. The mean age of the participants was 10.41 ± 2.224 years. The patients who were overweight or obese had higher liver enzyme and gamma-glutamyl transferase levels. Table 1 summarizes the baseline demographic and clinical information of the participants in our study.
| Variables | Cases | Controls | P-Value |
|---|---|---|---|
| Sex | > 0.99 | ||
| Male | 29 (58.0) | 29 (58.0) | |
| Female | 21(42.0) | 21(42.0) | |
| Age | 10.41 ± 2.224 | 10.41 ± 2.224 | > 0.99 |
| BMI percentile | |||
| Lab studies | 89.40 ± 3.326 | 44.08 ± 21.534 | < 0.001 |
| AST | 34.24 ± 15.663 | 11.08 ± 1.766 | < 0.001 |
| ALT | 52.33 ± 29.355 | 16.96 ± 8.517 | < 0.001 |
| ALP | 654.90 ± 258.390 | 599.14 ± 238.905 | 0.140 |
| GGT | 25.74 ± 8.278 | 16.36 ± 6.090 | < 0.001 |
Abbreviations: BMI: Body Mass Index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase.
a Values are presented as No. (%) or mean ± SD.
The patients underwent comprehensive abdominopelvic ultrasonography, which revealed a prevalence of 34% (95% CI: 21.2% - 48.8%) for NAFLD in the case group and a prevalence of 4.0% (95% CI: 0.5% - 13.7%) in the control group. In the assessment of NAFLD grading, overweight and obese participants experienced higher severities of NAFLD compared to their normal BMI counterparts. The diagnosis of NAFLD was again made by the lab data with a cutoff value of 50 IU/L for ALT and AST, resulting in a prevalence of 58.0% for NAFLD in overweight and obese children. The kappa measure of agreement was calculated for both groups to evaluate the agreement of sonographic and laboratory diagnosis of NAFLD. In obese children, the kappa measure of agreement was only 0.239 and non-significant, but this measurement in normal-BMI children was 1.000 and significant. Table 2 summarizes the comparison of the two groups regarding the prevalence of NAFLD.
| Non-alcoholic Fatty Liver Disease (NAFLD) | Case Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sonographic | Lab Data | Κ Measure | Sonographic | Lab Data | Κ Measure | |||||
| N (%) | 95%CI | N (%) | 95%CI | N (%) | 95%CI | N (%) | 95%CI | |||
| Total | 17 (34.0) | 21.2 - 48.8 | 29 (58.0) | 43.2 - 71.8 | 0.239 (P = 0.058) | 2 (4.0) | 0.5 - 13.7 | 2 (4.0) | 0.5 - 13.7 | 1.000 (P < 0.001) |
| Mild | 12 (24.0) | 13.1 - 38.2 | - | - | - | 2 (4.0) | 0.5 - 13.7 | - | - | - |
| Moderate | 3 (6.0) | 1.3 - 16.5 | - | - | - | 0 (0.0) | 0.0 - 7.1 | - | - | - |
| Severe | 2 (4.0) | 0.5 - 13.7 | - | - | - | 0 (0.0) | 0.0 - 7.1 | - | - | - |
In this study population, liver enzymes and gamma-glutamyl transferase were compared among the patients with and without NAFLD. In obese children, patients with NAFLD had significantly higher AST levels (43.06 ± 21.510 vs. 29.96 ± 8.605, P = 0.015), an association that was also seen in the total population. In obese children and the total population, NAFLD patients also had significantly higher ALT levels (41.72 ± 18.464 vs. 72.29 ± 35.721, P < 0.001 in the obese children and 26.03 ± 17.584 vs. 70.47 ± 34.248, P < 0.001 in the total population). The association between GGT and NAFLD was observed in obese children and the total population as well (P = 0.025 and P < 0.001, respectively). Table 3 summarizes the association between NAFLD and laboratory values.
| Variables | NAFLD vs. no NAFLD in Obese Children | NAFLD vs. no NAFLD in Total Population | ||||
|---|---|---|---|---|---|---|
| No NAFLD | NAFLD | P-Value | No NAFLD | NAFLD | P-Value | |
| AST | 29.96 ± 8.605 | 43.06 ± 21.510 | 0.004 | 18.68 ± 10.907 | 39.89 ± 22.385 | < 0.001 |
| ALT | 41.72 ± 18.464 | 72.29 ± 35.721 | < 0.001 | 26.03 ± 17.584 | 70.47 ± 34.248 | < 0.001 |
| ALP | 655.00 ± 279.646 | 669.41 ± 225.976 | 0.845 | 625.81 ± 257.087 | 645.35 ± 225.762 | 0.743 |
| GGT | 22.97 ± 7.577 | 28.65 ± 6.982 | 0.025 | 18.91 ± 7.422 | 27.95 ± 7.291 | < 0.001 |
Abbreviations: NAFLD, non-alcoholic fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase.
a Values are presented as mean ± SD.
For the assessment of the sonographic grading of NAFLD and laboratory studies in children, Spearman’s correlation was utilized, which showed a significant direct correlation between AST, ALT, GGT, and BMI percentile with NAFLD grading in overweight and obese children (P = 0.011, 0.001, 0.007, and < 0.001, respectively) and a direct correlation between the same variables with NAFLD grading in the total population (P < 0.001 for all four lab values). Table 4 summarizes the correlation between NAFLD grading and lab studies.
| Variables | NAFLD Grade in Obese Children | NAFLD Grade in Total Population | ||
|---|---|---|---|---|
| Spearman’s ρ | P-Value | Spearman’s ρ | P-Value | |
| AST | 0.362 | 0.011 | 0.413 | < 0.001 |
| ALT | 0.446 | 0.001 | 0.554 | < 0.001 |
| ALP | - 0.79 | 0.587 | - 0.013 | 0.901 |
| GGT | 0.379 | 0.007 | 0.427 | < 0.001 |
| BMI percentile | 0.527 | < 0.001 | 0.522 | < 0.001 |
Abbreviations: NAFLD, non-alcoholic fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase, ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; BMI, Body Mass Index.
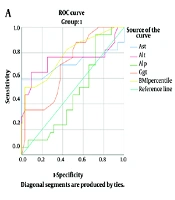
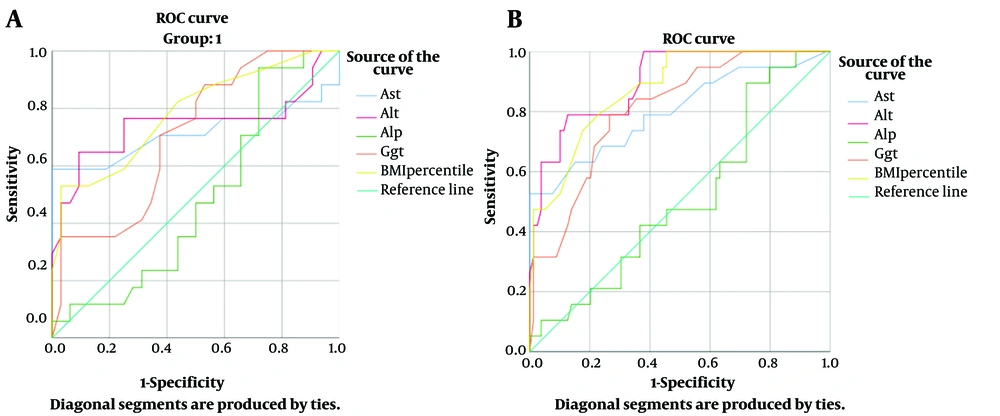
Furthermore, ROC curves for the lab values were drawn for the total population and obese children. Figure 1 shows the ROC curves of the diagnosis of NAFLD based on various lab values. The curves for AST, ALT, GGT, and BMI percentile were significant for the prediction of NAFLD. Aspartate aminotransferase seemed to be the most superior lab value to evaluate in patients suspected of NAFLD, as in obese children and in the total population, AST with a cut-off value of 43.5 had 100% specificity and positive predictive value for NAFLD. The optimal cut - off values of the lab values are summarized in Table 5.
| Variables | Obese Children | Total Population | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | P-Value | Cut-off | Sensitivity | Specificity | PPV | NPV | AUC | P-Value | Cut-off | Sensitivity | Specificity | PPV | NPV | |
| AST | 0.713 | 0.015 | 43.5 | 58.8 | 100 | 100 | 82.5 | 0.800 | < 0.001 | 43.5 | 52.6 | 100 | 100 | 90 |
| ALT | 0.744 | 0.005 | 65.0 | 64.7 | 91.6 | 78.6 | 83.3 | 0.899 | < 0.001 | 62.5 | 63.2 | 96.2 | 80 | 91.8 |
| ALP | 0.483 | 0.842 | N/A | N/A | N/A | N/A | N/A | 0.509 | 0.907 | N/A | N/A | N/A | N/A | N/A |
| GGT | 0.705 | 0.019 | 33.5 | 35.3 | 96.9 | 85.7 | 74.4 | 0.807 | < 0.001 | 29.5 | 31.6 | 91.1 | 46.2 | 85.1 |
| BMI percentile | 0.784 | 0.001 | 92.5 | 52.9 | 96.9 | 90.0 | 80.0 | 0.874 | < 0.001 | 90.5 | 47.4 | 98.7 | 75.0 | 88.6 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; BMI, Body Mass Index.
a Values are presented as No. (%).
5. Discussion
In this study, a total of 100 children, half of whom were obese or overweight, were evaluated regarding their liver enzymes and sonographic evidence of NAFLD. We found that the prevalence of NAFLD in normal-BMI children was 4.0% (95% CI: 0.5% - 13.7%), while the prevalence of this disease among overweight and obese children was 34.0% (95% CI: 21.2% - 48.8%). This indicates that overweight and obese children had a relative risk of about 8.5 for the development of NAFLD. In the global pediatric population, the prevalence of NAFLD is estimated to be between five and ten percent (18). Considering a national prevalence of about 11% for childhood obesity (19), the findings of our study align with previous studies evaluating the prevalence of NAFLD in children. Since pediatric NAFLD patients are at higher risk for cardiovascular mortality later in life, thorough evaluation of this condition and proper management of this disease and its sequelae seem to be important for healthcare policymakers (20).
Since the gold standard for diagnosing NAFLD and NASH consists of the invasive procedure of a liver biopsy, finding an alternative to this approach would increase the willingness of patients to undergo evaluation for this condition. Non-invasive diagnostic tools can also act as screening measures that suggest further steps in the NAFLD work-up. For this purpose, ultrasonography has been widely used, as it can show fatty infiltration of the hepatic parenchyma (21). However, the association between sonographic findings and lab studies has not been comprehensively studied. In this study, we used ultrasonography and laboratory studies as diagnostic methods for NAFLD. In the overweight and obese population, the agreement between the two methods was poor. The patients with NAFLD showed higher levels of alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase enzymes. The difference between NAFLD patients and normal children was more prominent when normal-BMI and high - BMI children were included in the comparison. This might stem from the fact that such enzymes might be influenced by other conditions seen in obesity, not only NAFLD (22). For example, obese and overweight children are more likely to have obesity-related hormonal disorders, such as polycystic ovary syndrome, which increases ALT levels (23, 24). Furthermore, the adipose tissue in obese children secretes pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), leptin, resistin, and adipokines, all of which increase oxidative stress in the hepatic parenchyma, leading to increased liver enzymes (25).
In the assessment of the correlation between lab studies and the degree of NAFLD, Spearman’s correlation test was used. The most important correlating factors were BMI percentile (ρ = 0.527, P < 0.001 in the total population, and ρ = 0.522, P < 0.001 in obese children) and ALT (ρ = 0.446, P = 0.001 in the total population and ρ = 0.554, P < 0.001 in obese children). In a study by Mansour-Ghanaei et al., performed on 950 participants of the PERSIAN Guilan cohort study, they assessed the association between liver enzymes and NAFLD and found that ALT, AST, and GGT were associated with this condition, with ALT being the most strongly correlated factor. ALP was not associated with this condition (26). The findings of our study align with those of the PERSIAN Guilan cohort study.
In a study by Khodadoostan et al., performed on 109 adult patients with NAFLD, the results of liver biopsy were compared with liver enzymes and ultrasonography findings. They found that the steatosis level in histopathology was associated with sonography findings, but a direct correlation was not found between liver enzymes and the steatosis level. They proposed that sonographic findings, unlike liver enzymes, might be useful in screening patients with NAFLD (27). These findings align with our results.
We also evaluated the optimal cutoff points for each laboratory parameter for the diagnosis of NAFLD. In this study, the BMI percentile outperformed other variables by having the highest area under the curve (AUC). However, a cutoff value of 43 IU/mL for AST had a positive predictive value of 100% for the diagnosis of NAFLD, making it more practical. Multiple models have incorporated various parameters like the liver function panel to evaluate the diagnosis of NAFLD. For example, Palekar et al. used AST, the AST/ALT ratio, BMI, age, and fasting insulin for a model that had an AUC of 0.76 (28). Angulo et al. also used age, history of hyperglycemia, BMI, platelet count, albumin levels, and the AST/ALT ratio for the diagnosis of liver fibrosis following NAFLD (29). In our study, the liver panel alone could indicate NAFLD with good precision.
The main limitation of this study was the relatively low number of patients and the inability to perform more advanced imaging like magnetic resonance elastography. Additionally, we could not correlate the results of sonography with liver fibrosis as assessed by histology. For further research, prospective studies with liver biopsy evaluations are suggested to measure the sensitivity, specificity, positive predictive value, and negative predictive value of sonography.
5.1. Conclusions
In this study, the prevalence of NAFLD in the normal-BMI pediatric population was 4.0%, but overweight and obese children were 8.5 times more likely to have NAFLD. Pediatric patients with sonographically diagnosed NAFLD had significantly higher levels of ALT, AST, and GGT, but no association was found between NAFLD and alkaline phosphatase. There was a low kappa measure of agreement between the sonographic and laboratory diagnosis of NAFLD in children, especially in obese and overweight patients. Implementing only lab values as a screening test for NAFLD seems unrealistic; therefore, sonographic evaluations should be integrated into the diagnostic evaluations of such patients.