1. Background
Lead, the most abundant heavy metal found in the terrestrial crust, poses serious health issues when ingested or absorbed through the skin (1). This problem has been recognized by physicians since two centuries before Christ when lead was used as a sweetener for wine. Nowadays, most lead toxicity is believed to result from environmental exposure to toxic substances such as industrial colors and cosmetics (2).
Lead is known to deteriorate neural signaling in the brain due to its competition with calcium for entrance into neurons (3). It can also interfere with the cellular respiratory cycle in mitochondria, increase the permeability of the blood-brain barrier, and affect vascular beds, leading to cerebral edema and intracerebral hemorrhage (4, 5). This condition can present with peripheral neuropathy, diminished levels of consciousness, weakness or paralysis, abdominal pain, and fine tremors (6-8). Lead negatively impacts fertility and acts as a teratogen in humans (9, 10). Moreover, animal studies have shown its carcinogenic effects (11). The toxic effects of lead pose a greater risk to pediatric patients, who may initially present with arthralgia and abdominal pain, potentially progressing to encephalopathy (12).
The most common sources of lead poisoning, especially in the Middle East and Northern Africa region, are lead-containing paints, lead-polluted water, food, soil, and air. Cultural habits, residential status, cooking accessibility, smoking, and pica phenomena also contribute to lead poisoning cases. According to the World Health Organization, lead toxicity was responsible for 21.7 million years of life lost in 2019, comprising about one-third of the global burden of idiopathic intellectual disabilities (13). Approximately 1.5% of total annual mortality is believed to be due to lead toxicity (14, 15). More than 500 million dollars are spent annually by the American government to prevent lead poisoning in the United States, yet this issue remains a significant health hazard worldwide, with about 800 million children having lead levels above 5 µg/dL (16, 17). Since these toxic levels of lead can have lasting effects on children’s academic and productive activities, proper evaluation of this toxidrome in regional studies is essential (13, 18).
2. Objectives
Thus, in the current study, we aimed to evaluate the prevalence of lead toxicity in children presenting with abdominal pain in Southern Iran.
3. Methods
This study followed a case-control protocol, evaluating serum lead levels in 50 pediatric patients with abdominal pain and 50 healthy counterparts. The inclusion criteria were patients younger than 18 years old with persistent abdominal pain for at least two months who had normal imaging and ultrasound results. Exclusion criteria included patients older than 18 years, those with abdominal pain for less than two months, nephrolithiasis, hepatosplenomegaly, gallstones, inflammatory bowel disease, abdominal pains requiring hospitalization, and unwillingness of parents or legal guardians to participate in the study.
After obtaining written informed consent from the parents of the study subjects, demographic and clinical information, as well as environmental exposure to lead, was collected. Additionally, 5 cc of blood was drawn from the patients for serum lead level assessment using the spectrophotometry method.
The data were analyzed using IBM SPSS version 26.0. Qualitative variables were reported as frequency and percentage, while quantitative variables were reported as mean and standard deviation. The prevalence of lead toxicity was reported for each group, with a 95% confidence interval calculated using a one-sample binomial test. Qualitative variables were compared between the two groups using a chi-squared test. Quantitative variables were compared using the independent two-sample t-test and the Mann-Whitney U test, as appropriate. P-values less than 0.05 were considered statistically significant.
This study was conducted in accordance with the Helsinki Declaration on ethics in biomedical research and was approved by the Committee of Ethics in Biomedical Research of Jahrom University of Medical Sciences under the code: IR.JUMS.REC.1403.005.
4. Results
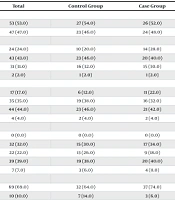
A total of 100 participants were included in the study, with 50 in the case group and 50 in the control group. The mean age of participants was 8.70 ± 2.845 years in the control group and 8.30 ± 1.974 years in the case group. Additionally, 54.0% of participants in the control group and 52.0% in the case group were male. There was no statistically significant difference between the two groups regarding baseline demographic information. Serum lead levels were measured in both groups. The mean serum lead level in the control group was 1.54 ± 0.693 µg/dL, while in the case group, it was 2.73 ± 2.547 µg/dL, which was significantly higher. Table 1 summarizes the comparison of the case and control groups regarding their demographic characteristics.
| Variables | Total | Control Group | Case Group | P-Value |
|---|---|---|---|---|
| Sex | 0.841 | |||
| Male | 53 (53.0) | 27 (54.0) | 26 (52.0) | |
| Female | 47 (47.0) | 23 (46.0) | 24 (48.0) | |
| Father’s education | 0.908 | |||
| Less than high school diploma | 24 (24.0) | 10 (20.0) | 14 (28.0) | |
| High school diploma | 43 (43.0) | 23 (46.0) | 20 (40.0) | |
| Associate or bachelor’s degree | 31 (31.0) | 16 (32.0) | 15 (30.0) | |
| Master’s degree or higher | 2 (2.0) | 1 (2.0) | 1 (2.0) | |
| Mother’s education | 0.611 | |||
| Less than high school diploma | 17 (17.0) | 6 (12.0) | 11 (22.0) | |
| High school diploma | 35 (35.0) | 19 (38.0) | 16 (32.0) | |
| Associate or bachelor’s degree | 44 (44.0) | 23 (46.0) | 21 (42.0) | |
| Master’s degree or higher | 4 (4.0) | 2 (4.0) | 2 (4.0) | |
| Father’soccupation | 0.796 | |||
| Unemployed | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Self-employed | 32 (32.0) | 15 (30.0) | 17 (34.0) | |
| Clerk or healthcare worker | 22 (22.0) | 13 (26.0) | 9 (18.0) | |
| Lay worker | 39 (39.0) | 19 (38.0) | 20 (40.0) | |
| Others | 7 (7.0) | 3 (6.0) | 4 (8.0) | |
| Mother’soccupation | 0.366 | |||
| Unemployed | 69 (69.0) | 32 (64.0) | 37 (74.0) | |
| Self-employed | 10 (10.0) | 7 (14.0) | 3 (6.0) | |
| Clerk or healthcare worker | 21 (21.0) | 11 (22.0) | 10 (20.0) | |
| Lay worker | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Others | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Age | 8.50 ± 2.444 | 8.70 ± 2.845 | 8.30 ± 1.974 | 0.416 |
| Weight | 30.24 ± 8.758 | 33.34 ± 8.973 | 27.14 ± 7.407 | < 0.001 |
| Weight percentile | 60.44 ± 31.081 | 75.69 ± 20.716 | 45.18 ± 32.371 | < 0.001 |
| Height | 127.04 ± 14.010 | 128.16 ± 15.756 | 125.92 ± 12.073 | 0.595 |
| Height percentile | 35.13 ± 23.258 | 36.64 ± 20.393 | 33.62 ± 25.930 | 0.252 |
| BMI | 18.45 ± 2.829 | 19.97 ± 1.659 | 16.94 ± 2.952 | < 0.001 |
| Serum lead level | 2.13 ± 1.950 | 1.54 ± 0.693 | 2.73 ± 2.547 | 0.002 |
Baseline Demographic and Clinical Information of the Patients a
Serum lead levels were categorized as high based on various definitions: Above 2 µg/dL (as stated by the laboratory), 3.5 µg/dL (as advised by the American Center for Disease Control and Prevention), 5 µg/dL, and 10 µg/dL (previous cut-off values for serum lead levels). Using the current advised cut-off value, six patients (12.0%) in the case group (95% CI: 4.5% - 24.3%) were found to have lead toxicity, while no participants in the control group showed this problem. Additionally, two patients (4.0%) in the case group had serum lead levels higher than 10 µg/dL. Table 2 summarizes the prevalence of lead toxicity in the study population.
| Reference Range (µg/dL) | Control Group | Case Group | P-Value | ||
|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | ||
| 2 | 7 (14.0) | 5.8 - 26.7 | 24 (48.0) | 33.7 - 62.6 | < 0.001 |
| 3.5 | 0 (0.0) | 0.0 - 7.1 | 6 (12.0) | 4.5 - 24.3 | 0.027 |
| 5 | 0 (0.0) | 0.0 - 7.1 | 4 (8.0) | 2.2 - 19.2 | 0.117 |
| 10 | 0 (0.0) | 0.0 - 7.1 | 2 (4.0) | 0.5 - 13.7 | 0.495 |
Prevalence of Lead Toxicity in the Study Population with Various Cut-off Values
Patients with serum lead levels above 3.5 µg/dL were compared to those with lower lead levels. There was no significant difference between patients with lead toxicity and those with normal lead levels regarding age, weight, height, or parental education and occupation. Although patients with lead toxicity had a longer duration of abdominal pain (28.00 ± 13.856 months vs. 17.54 ± 15.681 months), this difference was not statistically significant. Table 3 summarizes the comparison of patients with lead toxicity with those having normal serum lead levels.
| Variables | Lead Level > 3.5 µg/dL | Lead Level < 3.5 µg/dL | P-Value |
|---|---|---|---|
| Gender | 0.917 | ||
| Male | 3 (50.0) | 23 (52.3) | |
| Female | 3 (50.0) | 21 (47.7) | |
| Father’s education | 0.886 | ||
| Less than high school diploma | 1 (16.7) | 13 (29.5) | |
| High school diploma | 3 (50.0) | 17 (38.6) | |
| Associate or bachelor’s degree | 2 (33.3) | 13 (29.5) | |
| Master’s degree or higher | 0 (0.0) | 1 (2.3) | |
| Mother’s education | 0.493 | ||
| Less than high school diploma | 2 (33.3) | 9 (20.5) | |
| High school diploma | 3 (50.0) | 13 (29.5) | |
| Associate or bachelor’s degree | 1 (16.7) | 20 (45.5) | |
| Master’s degree or higher | 0 (0.0) | 2 (4.5) | |
| Father’s occupation | 0.058 | ||
| Unemployed | 0 (0.0) | 0 (0.0) | |
| Self-employed | 1 (16.7) | 16 (36.4) | |
| Clerk or healthcare worker | 0 (0.0) | 9 (20.5) | |
| Lay worker | 3 (50.0) | 17 (38.6) | |
| Others | 2 (33.3) | 2 (4.5) | |
| Unemployed | 5 (83.3) | 32 (72.7) | |
| Mother’s occupation | 0.768 | ||
| Self-employed | 0 (0.0) | 3 (6.8) | |
| Clerk or healthcare worker | 1 (16.7) | 9 (20.5) | |
| Lay worker | 0 (0.0) | 0 (0.0) | |
| Others | 0 (0.0) | 0 (0.0) | |
| Age | 8.08 ± 2.010 | 8.33 ± 1.991 | 0.787 |
| Weight | 26.67 ± 13.852 | 27.20 ± 6.338 | 0.297 |
| Weight percentile | 33.98 ± 37.320 | 46.71 ± 31.816 | 0.455 |
| Height | 124.00 ± 15.113 | 126.18 ± 11.787 | 0.511 |
| Height percentile | 28.86 ± 31.354 | 34.27 ± 25.461 | 0.404 |
| BMI | 16.37 ± 3.845 | 17.01 ± 2.857 | 0.709 |
| Duration of disease (months) | 28.00 ± 13.856 | 17.54 ± 15.681 | 0.320 |
Comparison of Patients with Lead Toxicity with the Ones with Normal Serum Lead Levels a
Among the patients with high serum lead levels, only one patient had exposure to industrial paint, and one other patient had exposure to repairing instruments. No other patients had exposure to lead toxicants such as cosmetics, batteries, printers, narcotics, or wastewater. No patient showed pica, but one patient with a serum lead level of 13.7 µg/dL exhibited neurologic signs of lethargy and attention deficit. No patients exhibited signs of cognitive or behavioral problems, ataxia, seizures, coma, hearing loss, memory loss, or weakness in extremities.
5. Discussion
In this study, the prevalence of lead toxicity in pediatric patients with chronic abdominal pain was assessed in the population of Southern Iran. We found that 12.0% of the patients with chronic abdominal pain had serum lead levels above 3.5 µg/dL. Until 2012, the American Center for Disease Control and Prevention recommended a cutoff value of 10 µg/dL in adults and 5 µg/dL in children to be considered elevated serum lead levels. However, in 2021, the CDC lowered the reference range to 3.5 µg/dL, equivalent to the 97.5th percentile in American children aged 1 - 5 years (19, 20).
Various cutoff values were examined for serum lead levels. We found that lower reference ranges resulted in a significant difference between the case and control groups regarding lead toxicity, but higher levels did not show a difference since the prevalence in the case group was low. Thus, our study confirms that lowering the reference range for normal blood lead levels increases the diagnostic and prognostic accuracy of its measurement.
Patients with lead poisoning exhibit a range of signs and symptoms from being asymptomatic to experiencing behavioral changes, headaches, anemia, abdominal pain, and encephalopathy with increasing levels of lead in the blood. Neuropsychiatric manifestations usually occur more intensely with acute exposures to lead, while abdominal pain and gastrointestinal manifestations typically follow more chronic exposures. Children are at higher risk for chronic exposure to lead due to their playfulness, which might increase the risk of ingesting lead-contaminated objects (21).
Lead can be stored in bones, teeth, brain tissue, spleen, kidneys, liver, and lungs when contacted for a prolonged duration. Its storage in solid organs, especially bones, increases its half-life, making the signs and symptoms more chronic (22). In our study, we compared the duration of symptoms in children with elevated and normal serum lead levels. We found that although patients with elevated serum lead levels suffered from more chronic symptoms, the difference between the two groups was not statistically significant. This apparent contradiction might arise from the low number of patients with lead toxicity; thus, further studies specifically on patients with elevated serum lead levels are suggested.
We found that patients with chronic abdominal pain were more likely to have lead toxicity. In a study by Afzali et al., one hundred and twenty adult patients with abdominal pain were evaluated, and those with and without possible lead exposure were compared. They found that both groups were not significantly different regarding high blood lead levels (23). Similarly, exposure to environmental factors in our study was not significantly different between the case and control groups. This finding may indicate that adequate exposure to lead contamination is not reliably assessed using questionnaires. Further public health examinations are needed for better precision in these studies.
The patients with the highest serum lead levels had parents working in painting and repairing industries, but risk factors for lead toxicity were not identified in other patients. In light of these findings and the fact that lead exposure can occur via soil, water, and air, investigating the environment in southern Iran is important. Additionally, gasoline refineries, residential paints, and petrochemical industries should be thoroughly evaluated for their lead-contaminating processes to decrease the public health burden of this element.
The parents of the patients with higher lead levels had lower education levels, but the difference between the two groups was not significant. This might be due to the fact that lead-exposing occupations, such as industrial painting or repair industries, are considered low-skilled jobs not requiring high levels of academic education. In a study by Ahmed Mokhtar Abo-Elfotoh et al. performed on 98 pediatric patients with lead toxicity and 643 healthy counterparts, they found that patients with lead toxicity were more likely to be of low social status, live in rural areas, have parents with lower levels of education, and have fathers working in labor-intensive jobs. The patients also had older mothers, and their fathers were more likely to be smokers; the patients played outdoors more frequently and were less likely to perform proper hand hygiene (24).
5.1. Conclusions
In this study, we found that the prevalence of lead toxicity among patients with chronic abdominal pain was 12.0% (95% CI: 4.5% - 24.3%), which was significantly higher than in the pediatric population without abdominal pain. However, further environmental studies are needed to evaluate lead-contaminated resources.