1. Background
Hyperleukocytosis (HL) is an oncologic emergency that can lead to early morbidity and mortality in children with acute lymphoblastic leukemia (ALL) (1, 2). Hyperleukocytosis is defined as a leukocyte count greater than 100,000 cells/mm³ and is present in 16.6% to 22.6% of patients at the time of acute leukemia diagnosis (1, 3). Hyperleukocytosis is associated with younger age, T-ALL subtype, and BCR-ABL rearrangements (4). In pediatric acute leukemia, HL is more common in ALL than in acute myeloid leukemia (AML) (5). However, children with hyperleukocytic AML are more likely to develop symptomatic leukostasis and metabolic changes related to tumor lysis (6, 7).
Patients with HL face an increased risk of developing symptomatic leukostasis (7), affecting the lungs, central nervous system, gastrointestinal tract, and cardiovascular systems. Hyperleukocytosis can lead to life-threatening complications such as intracerebral hemorrhage or pulmonary stasis, resulting in death (8-10). Treatment for HL and leukostasis involves intravenous hydration, chemotherapy (CT), and leukapheresis (LPH) (9, 11). Management of HL-associated tumor lysis includes the use of allopurinol or rasburicase for hyperuricemia, correction of electrolyte imbalances, and renal replacement therapy (8).
Leukapheresis, a procedure that rapidly reduces leukocyte count, is effective for cytoreduction but remains controversial. Its impact on reducing mortality is unclear (2, 8). Although LPH reduces circulating blast cells, the majority of leukemia cells reside in the bone marrow, limiting the overall effect. There are no established guidelines for LPH use, though it is often used as an adjunct to CT in cases of symptomatic leukostasis to prevent further metabolic derangements. Many patients receive concurrent CT (hydroxyurea or low-dose cytarabine), making it difficult to evaluate LPH's independent effects.
Hyperleukocytosis is an oncologic emergency due to its life-threatening complications, and LPH has been used for leukoreduction in patients with HL. However, the absence of clinical guidelines and randomized controlled trials contributes to the ongoing debate over LPH's use (12-14). Reported outcomes range from no improvement in survival with LPH to improved early survival (15-17). Some studies caution against using LPH, while others report that it is safe and well-tolerated (16-19). Given the cost, resource use, and potential complications of LPH, randomized studies are needed to evaluate its clinical efficacy.
2. Objectives
This study aimed to describe the characteristics, clinical course, and outcomes of pediatric patients with acute leukemia and HL, comparing those who received LPH to those who did not.
3. Methods
3.1. Patients
A retrospective review was conducted of all previously untreated pediatric leukemia patients who presented to the Pediatric Hematology and Oncology Clinic with HL between 2017 and 2022. Data collection included diagnostic studies, laboratory values (WBC, hemoglobin, platelet count, lactate dehydrogenase, uric acid, creatinine, potassium, calcium, phosphorus), and interventions within 72 hours of diagnosis. Results of LPH, as well as short-term (30-day) survival outcomes, were also reviewed. Demographic and laboratory data were recorded within 72 hours of hospital admission. The study was approved by the Institutional Ethics Board (ethics board No: 2022.10.343), and informed consent was obtained from parents, with assent from patients when appropriate.
3.2. Definitions and Complications
Hyperleukocytosis was defined as a WBC count > 100,000 cells/mm³.
Leukostasis refers to symptomatic HL, a medical emergency requiring prompt recognition and treatment to prevent renal and respiratory failure or intracranial hemorrhage.
Tumor lysis syndrome (TLS) was defined as the presence of at least two of the following metabolic derangements within three days of leukemia-directed therapy: (1) hyperuricemia, (2) hyperkalemia, (3) hyperphosphatemia, and hypocalcemia (laboratory TLS). Clinical TLS was defined as laboratory TLS plus increased creatinine, seizures, cardiac dysrhythmia, or death (17). Hyperkalemia was defined as potassium > 5.5 mmol/L, hyperphosphatemia as > 5.2 mg/dL, hypocalcemia as < 8.5 mg/dL, hypermagnesemia as > 3.0 mg/dL, and hyperuricemia as > 7.0 mg/dL.
3.3. Leukapheresis and Cytoreduction with Chemotherapy
The decision to perform LPH was made on a case-by-case basis by the attending pediatric oncologist in consultation with the ICU physician. LPH was recorded in patients with WBC > 300,000 cells/mm³ or symptomatic HL. In the non-LPH group (n = 32), one patient had symptomatic HL, and two patients had WBC > 300,000 cells/mm³. Differences in practice among pediatric hematology and oncology specialists at our hospital, who trained at different centers, contributed to variability in management. Adverse events during the first 30 days post-diagnosis, including hemorrhagic, neurologic, renal, and respiratory complications, were reviewed. The mean time from presentation to CT initiation was 17 - 18 hours. The concurrent use of hydroxyurea, steroids, or low-dose cytarabine may have confounded the evaluation of LPH's independent effects.
3.4. Statistical Analysis
Statistical analysis was conducted using IBM SPSS statistics 22. The Kolmogorov-Smirnov test was used to assess normality, and parameters did not follow a normal distribution. Descriptive statistics (minimum, maximum, mean, standard deviation, median, frequency) were calculated. Quantitative data were compared between groups using the Mann-Whitney U test for non-normally distributed parameters, while Fisher's exact chi-squared test was used for qualitative data comparisons. Multivariate analysis was conducted using logistic regression. Statistical significance was set at P < 0.05.
4. Results
4.1. Patient Characteristics
During the study period, 404 children were diagnosed with acute leukemia, of whom 41 (10.1%) had HL. The median age among the 41 patients with HL was 6 years (range 1 - 17 years), and 27 (65.9%) were male. Patients with HL were more likely to be male, have a T-cell immunophenotype, and present with elevated lactate dehydrogenase (LDH) and uric acid levels. Among these patients, 32 had acute lymphoblastic leukemia and 9 had acute myeloblastic leukemia. One patient had a rearrangement of BCR-ABL.
Nine of the 41 patients (22%) received LPH. The mean age in the LPH group was 7 years (range 2 - 13 years), compared to 6 years (range 1 - 17 years) in the non-LPH group. The majority of patients in both groups were male. Patients diagnosed with ALL were more common in both groups, with about half being T-ALL cases. In the LPH group, 6 patients had T-ALL, 2 had AML, and 1 had B-ALL. In the non-LPH group, there were 9 T-ALL cases, 7 AML cases, and 16 B-ALL cases (Table 1).
| Characteristics | LPH Group (n = 9) | Non-LPH Group (n = 32) | Total (N = 41) | P-Value |
|---|---|---|---|---|
| Age (y) | 7 (2 - 13) | 6 (1 - 17) | 6 (1 - 17) | 0.448 b |
| Gender | 0.692 c | |||
| Male | 7 (77.8) | 20 (62.5) | 27 (65.9) | |
| Female | 2 (22.2) | 12 (37.5) | 14 (34.1) | |
| Immunophenotype | 1.000 c | |||
| ALL | 7 (77.8) | 25 (78.1) | 32 (78) | |
| AML | 2 (22.2) | 7 (21.9) | 9 (22) | |
| Cytogenetics | 1.000 c | |||
| Abnormal | 0 | 1 (3.1) | 1 (2.4) | |
| Normal | 9 (100) | 31 (96.9) | 40 (97.6) | |
| Laboratory findings | ||||
| WBC (cells/mm3) | 560.000 (168.000 - 844.000) | 125.000 (101.000 - 430.000) | 136.000 (101.000 - 844.000) | 0.001 b,d |
| Uric acid | 6 (2.5 - 16) | 5.7 (1.8 - 19) | 5.8 (1.8 - 19) | 0.625 b |
| LDH | 3657 (715 - 50.003) | 823 (2.7 - 9.790) | 1158 (2.7 - 50003) | 0.002 b,d |
| Potassium | 3.6 (2.5 - 5) | 4.5 (3.1 - 10.3) | 4.2 (2.5 - 10.3) | 0.002 b,d |
| Phosphorus | 3.1 (1.7 - 5.5) | 4.3 (1.2 - 6.2) | 4.1 (1.2 - 6.2) | 0.014 b,d |
| Calcium | 9.2 (7.9 - 10.5) | 8.8 (3 - 10.5) | 9 (3 - 10.5) | 0.207 b |
| Findings, signs, and symptoms | ||||
| Neurologic leukostasis | 0 (0) | 0 (0) | 0 (0) | - |
| Pulmonary leukostasis | 1 (11.1) | 1 (3.1) | 2 (4.9) | 0.395 c |
| Tumor lysis syndrome | 2 (22.2) | 2 (6.3) | 4 (9.8) | 0.204 c |
| Treatment | ||||
| Rasburicase | 3 (33.3) | 3 (9.4) | 6 (14.6) | 0.107 c |
| Mean (range) time to CT (h) | 16.11±3.92 (17; 6 - 20) | 17.69±2.21 (18; 6 - 21) | 17.34±2.70 (18; 6 - 21) | 0.006 b,d |
| Initial stay in PICU | 9 (100) | 4 (12.5) | 13 (31.7) | 0.001 c, d |
| Mean (range) LOS PICU | 4.22±1.72 (4; 2 - 7) | 18.0±24.7 (6.5; 4 - 55) | 8.46±14.08 (4; 2 - 55) | 0.079 b |
| 30-day Mortality | 0 | 0 | 0 |
Characteristics of Patients a
There was no statistically significant difference between the LPH and non-LPH groups in terms of mean age, gender distribution, symptoms, immunophenotype, cytogenetics, uric acid, calcium levels, use of rasburicase, time to CT initiation, or intensive care unit (ICU) stay (P > 0.05) (Table 1). However, WBC levels were significantly higher in patients who underwent LPH compared to those who did not (P = 0.001). Additionally, LDH levels were significantly higher in LPH patients (P = 0.002), while potassium and phosphorus levels were significantly lower in LPH patients (P = 0.002). The rate of ICU admission was 100% in the LPH group, compared to 12.5% in the non-LPH group (P = 0.001). According to these results, high WBC count and elevated LDH levels are predictive factors for the need for LPH.
A backward stepwise logistic regression analysis showed that WBC, LDH, potassium, and phosphorus levels significantly predicted the need for LPH (P = 0.001), with a Nagelkerke R-squared value of 0.741, indicating a good explanatory power of the model (87.8%). The effect of WBC on LPH use was statistically significant (P = 0.006).
The median WBC count in the LPH group was 520,000 cells/mm³ (range 168,000–844,000 cells/mm³), compared to 158,000 cells/mm³ (range 101,000–430,000 cells/mm³) in the non-LPH group (P = 0.01). The time from presentation to CT initiation was similar in both groups (mean of 17 hours in the LPH group and 18 hours in the non-LPH group; P > 0.05). Tumor lysis syndrome (TLS) was observed in 2 patients (23%) in the LPH group and 2 patients (6.25%) in the non-LPH group (P > 0.05). All patients received intravenous hydration, with rasburicase treatment administered to 3 patients in each group, and allopurinol used for the remaining patients (Tables 1 and 2).
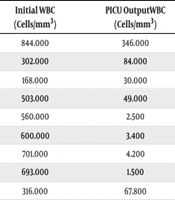
| No. | Age | Sex | Diagnosis | LSS | TLS | Initial WBC (Cells/mm3) | PICU Output WBC (Cells/mm3) | Time to CT, First 24 (h) | LOSPICU (Days) | Status (First 30 Days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | M | B-ALL | - | - | 844.000 | 346.000 | + | 3 | Alive |
| 2 | 13 | F | AML-M5 | - | - | 302.000 | 84.000 | + | 4 | Alive |
| 3 | 11 | M | AML-M2 | Dyspnea | - | 168.000 | 30.000 | + | 4 | Alive |
| 4 | 10 | M | T-ALL | - | - | 503.000 | 49.000 | + | 7 | Alive |
| 5 | 2 | M | T-ALL | - | + | 560.000 | 2.500 | + | 4 | Alive |
| 6 | 7 | M | T-ALL | - | - | 600.000 | 3.400 | + | 4 | Alive |
| 7 | 2 | M | T-ALL | - | - | 701.000 | 4.200 | + | 7 | Alive |
| 8 | 12 | F | T-ALL | - | + | 693.000 | 1.500 | + | 3 | Alive |
| 9 | 6 | M | T-ALL | - | - | 316.000 | 67.800 | + | 2 | Alive |
The Initial Presentation and Outcomes of Patients in the Leukapheresis Group
4.2. Symptoms of Leukostasis
No neurologic leukostasis (e.g., vision changes, altered mental status, intracranial hemorrhage) was seen in the LPH group, while one patient in the non-LPH group developed pulmonary leukostasis (e.g., dyspnea, hypoxia, respiratory failure) (P > 0.05). Clinical leukostasis data is summarized in Table 1.
4.3. Results of Leukapheresis
Leukapheresis was performed prophylactically in 8 patients, while 1 patient underwent therapeutic LPH due to dyspnea. On average, there was a decrease of 454,629 cells/mm³ in WBC count following LPH. The median WBC count before the first apheresis was 520,000 cells/mm³, which was reduced to a mean of 65,529 cells/mm³ after the procedure. The mean ICU stay for patients who underwent LPH was 2 days (range 2 - 7 days) (Table 2).
4.4. Mortality and Complications Outcomes
Leukapheresis was well tolerated, with no life-threatening complications observed in any patient. No early deaths occurred within the first 30 days after presentation in any of the patients (Table 3).
| Characteristics | All; N = 41 | LPH | P-Value | |
|---|---|---|---|---|
| Yes; n = 9 | No; n = 32 | |||
| Any | NS | |||
| Hemorrhagic/vascular | 0 (0) | 0 (0) | 0 (0) | NS |
| Renal | 0 (0) | 0 (0) | 0 (0) | NS |
| Respiratory | 2 (4.88) | 1 (11) | 1 (3) | NS |
| Neurologic | 0 (0) | 0 (0) | 0 (0) | NS |
Comparison of Pertinent Complications Among Acute Leukemia Patients Who Presented with Hyperleukocytosis (Leukapheresis vs. Non-leukapheresis) a
The median follow-up time for all patients was 21 months (range 3 - 70 months). In the non-LPH group, 2 patients died at 7 months and 1 at 11 months. One patient underwent bone marrow transplantation (BMT) for refractory disease at 16 months and is currently being followed at 21 months. One patient relapsed at 6 months and underwent BMT, and another relapsed at 24 months and is currently being followed at 57 months. In the LPH group, no patients died; 3 patients received BMT for refractory disease, and 1 patient underwent BMT for relapse at 3, 5, 8, and 14 months, respectively.
5. Discussion
Hyperleukocytosis is an oncological emergency in pediatric patients with acute leukemia. It has been shown that the risk of mortality increases when neurological/Pulmonary leukostasis develops (5, 8). Leukapheresis is recommended in leukostasis or when the WBC is elevated, but there is no definite consensus on the management of these patients. It has been suggested that LPH should be performed when the WBC exceeds 400,000 cells/mm3 (5). However, other authors suggested that LPH could not be applied to any patient, regardless of the WBC, since it could not be performed in this particular center and no patient loss was experienced, despite the inability to use LPH (20). Compared with patients who received LPH, the percentage decrease in leukocyte counts was greater among those who received CT (21). In the present study, the WBC of patients who underwent LPH was > 300,000 cells/mm3 with one exception. When the patients who did not have LPH were examined, the WBC was > 300,000 cells/mm3 in only two patients. When both the LPH and non-LPH groups were examined, no death was observed in the first month of follow-up and there were 32 (78.05%) patients in the non-LPH group. In addition, after LPH, the WBC decreased from a mean of 510,000 cells/mm3 to 65,000 cells/mm3. This improvement may be due to the prompt availability of platelet transfusion for thrombocytopenia, delayed packed red blood cell transfusion until resolution of HL, early initiation of low-dose cytoreductive CT, and careful management of TLS. Although LPH treatment appears effective and safe, treatment of HL is possible with only hydration support, administration of allopurinol/rasburicase, early initiation of treatment for the disease within the first 24 hours, and close clinical follow-up. LPH may be performed in patients with leukostasis, if it is not possible to start induction CT early (20).
Increasing age, male gender, and T-cell ALL phenotype are reported to be predictors for LPH (5, 16, 19, 22). In our study, male gender, and T-cell ALL phenotype were associated with an increased probability of LPH, in keeping with the literature. Seven (77.8%) of the 9 patients were male, and the T ALL rate was 67%. In our study, LDH levels were high in leukemia patients with HL and were significantly higher in the LPH group than in the other group. Elevated circulating LDH levels have been considered a marker of poor prognosis in oncology and have often been attributed to high tumor burden and cancer metabolism. Recent evidence suggests that elevated LDH levels may be independent of tumor burden and may have a negative predictive value, which may be helpful in guiding treatment strategies in immuno-oncology (23). Taken together, high LDH levels can also be considered a marker for LPH.
Studies have reported conflicting results regarding whether LPH delays the time to CT (7, 24). Given that the patient is receiving a therapeutic intervention, a prolonged time to CT would normally be expected in patients receiving LPH. Although LPH rapidly reduces the WBC count, it usually needs to be administered several times to achieve an effective reduction in the WBC count. In our study, there was no significant difference in the time to CT between patients who received LPH and those who did not. This may be due to the fact that the patient was awaiting flow cytometry results and concurrent cytoreduction therapy was started as soon as the diagnosis was confirmed.
The larger size of lymphoblasts compared to normal blood cells predisposes patients with HL to develop leukostasis (16). Hence, it is not surprising that children with acute leukemia and HL have a higher incidence of early adverse events, including death (25). The clinical symptoms of leukostasis increase the likelihood of requiring LPH (22). Only one patient who had LPH presented with a sign of leukostasis. This outcome likely reflects that physicians at our institution initiate LPH based directly in response to HL rather than directly in response to clinical scenarios. There were two patients with dyspnea as a symptom of leukostasis, one of whom underwent LPH. The patient who did not undergo LPH required 55 days in the PICU, while the patient who underwent LPH required only seven days in the PICU. The WBC in these two patients was 202,000 cells/mm3 and 168,000 cells/mm3, respectively. It should be noted that the patient who did not undergo LPH was an infant and had been diagnosed with AML, which may have contributed to the prolonged PICU stay. In our study, no neurological leukostasis was observed.
Complications related to LPH use have been reported, such as hemodynamic changes, electrolyte irregularities, bleeding due to anticoagulation use, and risks associated with central lines (14). However, no significant complications were reported in the present study, as in similar previous studies (13, 26). Lastly, the lack of complications among the studied subgroups of patients needs to be interpreted cautiously given the relatively small number of patients and events.
Among all HL patients, there were no deaths within the first 30 days, while 3 patients died during long-term follow-up in the group that did not receive LPH. Two of these patients died due to refractory disease (one with AML and one with T-cell ALL) and one died due to septic shock (with B-cell ALL). It was noted that 4 patients in the LPH group and 3 patients in the non-LPH group underwent BMT due to relapsed/refractory disease. Of the 7 patients who underwent BMT, all but 1 (diagnosed with B-cell ALL) were diagnosed with AML and T-cell ALL. The life expectancy of patients with T-cell ALL and AML was lower than that of patients with B-cell ALL. Febrile neutropenia is the most common cause of death in patients with leukemia. Although all patients who died were in the non-LPH group, it is not possible to attribute this result to non-LPH because the patient population is small and the factors affecting life expectancy in leukemia patients are multifactorial.
Current studies on the use of LPH in leukemia patients with HL have not resolved the question marks of previous studies, and no clear answer has been found to completely resolve the differences in opinion and practice between centers. Studies suggesting that LPH is not necessary have cited the lack of effect of LPH on early mortality (17, 27, 28). However, more recent studies have recommended the use of LPH. Zhang et al. emphasized that LPH rapidly eliminates leukocytes and corrects metabolic abnormalities, alleviates symptoms of leukostasis, and is well tolerated (22). There are reports that LPH eliminates the symptoms of leukostasis, TLS, and DIC, thereby reducing the leukemic cell burden without life-threatening risks (29, 30). The use of LPH has also been reported to be safe and well tolerated (18, 31). In one study, although there was no significant difference in survival in the LPH group, it was stated that LPH has a therapeutic role and is a safe and effective option (32). Because of the lack of consensus in the current studies, it does not seem possible to establish a practice guideline in the near future. All these recent studies show us that although LPH is effective, it is not possible to say clearly that the absence of LPH negatively affects survival rates. In our study, although the number of cases was limited, the use of LPH seems to be safe and at least survival rates were not decreased in patients without LPH. Another important reason for the different results in the studies may be other therapeutic factors such as steroids, CT, hydration, uricolysis/rasburicase used simultaneously with LPH and other genetic factors, biomarkers, pharmacokinetic and pharmacodynamic individual factors that we do not know.
In the management of patients with HL, prompt and coordinated intervention is required to assess the patient's risk and prevent complications. This study has shown that LPH is a procedure that provides an effective reduction in leukocyte count in patients presenting with acute leukemia with HL and has an acceptable side-effect profile. The effectiveness of LPH treatment is usually temporary. The number of blasts in the peripheral circulation may increase rapidly shortly after the procedure. The standard treatment for patients with acute leukemia with HL is supportive therapy and induction CT (16). To prevent rebound leukocytes and blasts, cytoreductive therapy, such as the use of hydroxyurea and/or induction CT, should be initiated rapidly (33).
The limitations of our study are the small cohort size and its retrospective design. In particular, the sample size in the LPH group is small because the retrospective nature of our study allows us to evaluate existing data. In general, LPH was administered to patients with WBC count > 300,000 or leukostasis symptoms, but 3 patients were excluded from these criteria. The fact that treatments such as hydration, steroid/CT, uricolysis/rasburicase were given simultaneously with LPH makes it difficult to evaluate the efficacy of LPH alone. Future prospectively designed studies with larger case groups may give us clearer results regarding the necessity of LPH in patients with HL.
5.1. Conclusions
The optimal management of symptomatic HL is still uncertain, and there are no randomized studies demonstrating one is superior to the other. Therefore, it is recommended that intensive CT should be implemented as quickly as possible in patients, in addition to supportive measures. Our results show no early deaths related to HL among these patients and suggest that LPH may not be necessary to reduce the occurrence of early adverse events in this population.