1. Background
Familial Mediterranean fever (FMF) is a hereditary autoinflammatory disorder characterized by recurrent episodes of fever and inflammation affecting the peritoneum, pleura, synovial membrane, and skin (1). Although it is more prevalent in the Mediterranean region and nearby areas, particularly among Jews, Turks, Arabs, and Armenians, the disease has global significance due to its widespread impact and the potential implications of population migration (1, 2).
Mutations in the MEFV gene, located on chromosome 16, are the primary cause of FMF. This gene encodes pyrin, also known as marenostrin, a protein involved in regulating inflammation. Over 300 distinct mutations have been identified in the MEFV gene, with M694V, M680I, V726A, and E148Q being the most common (3).
1.1. Pathogenesis
The M694V mutation is the most prevalent mutation in the MEFV gene in Türkiye, followed by M680I and V726A (4). These mutations play a central role in the pathogenesis of FMF by activating and triggering interleukin-1β (IL-1β) in the inflammatory cascade (1, 3).
Pyrin, the protein encoded by the MEFV gene, acts as a regulator to prevent excessive inflammation by inhibiting the activation of the inflammasome. In FMF, the mutated pyrin's reduced efficacy leads to uncontrolled inflammasome activation and overproduction of IL-1β, which drives the characteristic symptoms of FMF, such as recurrent fever, abdominal pain, chest pain, and joint inflammation (1, 3).
Although FMF is primarily a genetic disorder, the frequency and severity of inflammatory episodes can be influenced by environmental and physiological factors. Stress, infections, and physical exertion can potentially trigger or worsen FMF symptoms, although the precise mechanisms of these triggers remain unclear (5).
In patients with FMF and MEFV mutations, a minor trigger, such as emotional stress, leads to decreased phosphorylation of the mutated pyrin protein. As a result, a greater quantity of active, non-phosphorylated pyrin is produced, promoting the formation of the pyrin inflammasome and driving inflammation. The mutated pyrin protein in FMF gains the ability to function autonomously, independent of external stimuli such as toxins or infections. This process results in the secretion of IL-1, IL-18, and other inflammatory mediators, which promote chemotaxis and neutrophilia, ultimately triggering an FMF attack (6).
1.2. Clinical Manifestation
In the majority of cases, attacks begin before the age of 20, affecting 90% of patients. The onset of attacks is sudden, with durations ranging from 6 to 96 hours, and they resolve spontaneously. Severe abdominal pain may be accompanied by high fever during these episodes. It is common for patients to experience symptom-free intervals between attacks. Abdominal pain is present in 95% of observed cases, and clinical as well as pathological findings are consistent with typical peritonitis. The clinical presentation may resemble an acute abdomen, which could lead to appendectomy or laparotomy. Additionally, joint pain, muscle aches, chest pain, and arthritis are other common symptoms of the disease (5).
The most serious complication of FMF is amyloidosis, which primarily affects the kidneys (5, 7). However, due to early diagnosis and the widespread use of colchicine, the frequency of amyloidosis has significantly decreased (1, 8, 9).
Familial Mediterranean fever is commonly observed in the Mediterranean region, including Türkiye, as well as in the Middle East. Early recognition of the disease in children is crucial for preventing complications like amyloidosis, which can reduce both morbidity and mortality.
2. Objectives
Although studies have been published regarding disease-related mutations, additional research could provide further insights into the clinical implications of these mutations. The aim of this study was to investigate the potential clinical implications of the observed genetic mutations.
3. Methods
3.1. Study Design
The study included 326 patients diagnosed with FMF who underwent MEFV gene mutation analysis and received colchicine treatment from 2009 to 2013. The diagnosis of FMF was based on the criteria proposed by Yalcinkaya et al. for pediatric patients (10). Yalcinkaya et al.’s criteria have been suggested as an alternative to the Tel Hashomer criteria, which include five clinical features: Fever, abdominal pain, chest pain, arthritis, and a family history of FMF. According to these criteria, attacks lasting over 6 hours and meeting two or more of the five criteria confirm the diagnosis, with a sensitivity of 86.5% and a specificity of 93.6% (11).
Sixteen patients were not included in the study as FMF was ruled out clinically and colchicine treatment was discontinued. Additionally, eight patients were excluded due to unknown results in the MEFV gene mutation analysis. Therefore, a total of 302 patients were enrolled in the study.
A retrospective assessment of the patients' characteristics was performed using the hospital information management system. The collected data included patients' age, gender, height, weight, admission dates, personal and family history, complaints, clinical findings at admission, physical examination findings, laboratory results, MEFV gene mutation analysis results, complaints and clinical findings during follow-up evaluations, and details regarding the administered treatments.
The study received approval from the institutional research ethics committee (verdict number 17.12.2013/443). Prior to beginning the study, the parents of the children were thoroughly informed, and their consent was obtained. Data assessment was conducted personally by the principal investigator, who reviewed all patient data over a five-month period.
3.2. Procedures
The process of sequencing the MEFV gene in our center begins with isolating the genomic DNA. The next step involves amplifying the DNA through polymerase chain reaction (PCR). After amplification, the PCR products are purified before proceeding to the sequencing PCR. Once the sequencing PCR is completed, the sequencing products undergo a second purification process. Electrophoresis is then performed on the purified products using the 3130 xl Genetic Analyzer. Following electrophoresis, the data obtained is analyzed. Our institution employs PCR protocols to investigate the exon-2, exon-3, and exon-10 regions of the MEFV gene.
3.3. Bias
This study addresses several potential biases that may arise from using retrospective data from hospital information systems. Selection bias is a significant concern, as the data may primarily represent patients who regularly visit specific healthcare facilities, leading to findings that lack generalizability. Incomplete or inconsistent data entry can result in information bias, causing inaccuracies in the recorded information. Additionally, the results may be influenced by confounding variables since the study's retrospective design limits control over all influencing factors. Acknowledging these biases is essential for accurately interpreting the study’s conclusions and assessing the reliability of its findings.
Overall, the presence of potential biases limits the validity of conclusions drawn from retrospective studies that rely on hospital data. Due to these biases and incomplete information, it is necessary to interpret the findings with caution and encourage further research. Addressing these limitations in future studies can improve the reliability and applicability of the findings to broader populations.
Lastly, the study uses listwise deletion to manage missing data by analyzing only complete records. While this method ensures data consistency, it reduces the sample size and may introduce bias if the missing data are not missing completely at random (MCAR).
3.4. Statistical Analysis
The data was analyzed using SPSS software version 18, employing a comprehensive statistical analysis approach. Since the study sample consisted of more than 50 participants, the Kolmogorov-Smirnov test was used to determine if the continuous variables followed a normal distribution. Continuous variables were summarized as the mean and standard deviation (SD), while categorical variables were presented as counts and percentages (%) to describe the clinical and demographic characteristics. To compare normally distributed variables between two independent groups, the Student’s t-test was applied. For variables that did not follow a normal distribution, the Mann-Whitney U test was used. The chi-squared test assessed the association between categorical variables. Correlation, univariate, and multivariate logistic regression analyses were conducted to identify predictive factors for colchicine treatment resistance. Statistical significance was established with a P-value of less than 0.05.
In this study, missing data were addressed through listwise deletion, a method that includes only cases with complete data for all relevant variables in the analysis. The analysis focused on valid cases to ensure that all necessary data points were available. This approach ensures that the analyses are based on complete and consistent datasets.
4. Results
4.1. Demographic Characteristics
The study population consisted of 302 patients (157 females, 145 males) with a mean age of 142.60 ± 48.04 months. On average, patients were diagnosed at 94.42 ± 42.39 months, while the mean age of symptom onset was 25.96 ± 25.86 months. No significant gender differences were observed in terms of age at presentation, diagnosis, or onset of symptoms.
Among the patients, 106 (35.1%) reported consanguinity, with the majority (26.0%) being first-degree cousin marriages. Consanguinity rates did not significantly differ between males and females (P = 0.964). Additionally, 74 patients (24.5%) had a family history of FMF, and no significant gender difference was found (P = 0.285). Table 1 presents the demographic characteristics of the patients.
| Demographic Characteristics | Overall; (n = 302) | Female; (n = 157) | Male; (n = 145) | P-Value |
|---|---|---|---|---|
| Age (mo) | 142.60 ± 48.04 | 140.76 ± 48.10 | 144.59 ± 50.13 | 0.490 |
| Age at diagnosis (mo) | 94.42 ± 42.39 | 91.28 ± 41.38 | 97.81 ± 43.34 | 0.189 |
| Age of onset at complaints (mo) | 25.96 ± 25.86 | 25.10 ± 23.36 | 27.15 ± 29.13 | 0.641 |
| Consanguinity | 0.964 | |||
| None | 152 (58.9) | 74 (57.4) | 78 (51.3) | |
| 1st degree cousin | 67 (26.0) | 35 (27.1) | 32 (24.8) | |
| 2nd degree cousin | 14 (5.4) | 7 (5.4) | 7 (5.4) | |
| Distant relative | 25 (9.7) | 13 (10.1) | 12 (9.3) | |
| Family history of FMF | 0.285 | |||
| None | 193 (72.3) | 91 (67.9) | 102 (72.3) | |
| 1st degree cousin | 57 (21.3) | 33 (24.6) | 24 (18.0) | |
| 2nd degree cousin | 10 (3.7) | 7 (5.2) | 3 (2.3) | |
| Distant relative | 7 (2.6) | 3 (2.2) | 4 (3.0) |
Abbreviation: FMF, familial Mediterranean fever.
a Values are expressed as No. (%) or mean ± SD.
4.2. Clinical Features and Laboratory
Table 2 presents the clinical symptoms, signs, and complaints reported by the patients. Abdominal pain was the most common complaint, with a prevalence of 90.3%, followed by fever at 69.0% and arthralgia at 42.3%. Chest pain, headache, and arthritis were less frequently mentioned, with rates of 15.5%, 13.4%, and 10.0%, respectively. Among the 29 patients with arthritis, 86.2% (25 patients) experienced involvement of large joints, such as the wrist, elbow, knee, or ankle.
| Variables | Number of Patients a | Frequency (%) |
|---|---|---|
| Symptoms and signs | ||
| Abdominal pain | 290 | 262 (90.3) |
| Fever | 290 | 200 (69.0) |
| Arthralgia | 291 | 123 (42.3) |
| Chest pain | 291 | 45 (15.5) |
| Headache | 291 | 39 (13.4) |
| Arthritis | 291 | 29 (10.0) |
| Growth retardation | 296 | 27 (9.1) |
| Vomiting | 291 | 25 (8.6) |
| Myalgia | 291 | 23 (7.9) |
| Rash | 291 | 16 (5.5) |
| Lumbar pain | 291 | 15 (5.2) |
| Nocturnal enuresis | 291 | 10 (3.4) |
| Diurnal enuresis | 291 | 7 (2.4) |
| Pericarditis | 291 | 1 (0.3) |
| Biomarkers | ||
| CRP (> 5 mg/L) | 247 | 119 (48.2) |
| Fibrinogen (400 mg/dL) | 234 | 91 (38.9) |
| Erythrocytes sedimentation rate (> 15 mm/h) | 247 | 121 (49) |
| Increase in CRP, sedimentation, and fibrinogen simultaneously | 244 | 39 (16) |
a All patients with available data at admission.
The most frequent clinical observation at the time of admission was monthly attacks of fever and/or abdominal pain, reported by 31.0% of patients. The frequency of these attacks was four or more per month. Specifically, 27% (n = 27) of the patients experienced at least four fever attacks per month, while 24% (n = 31) reported at least four abdominal pain attacks per month.
Additional rheumatological conditions were also observed in FMF patients, with 10 individuals (3.4%) diagnosed with juvenile idiopathic arthritis (JIA), 8 individuals (2.6%) with Henoch-Schoenlein vasculitis (HSV), and 1 individual with polyarteritis nodosa (PAN).
Out of 285 patients, 76 (26.6%) were diagnosed with anemia at the time of admission, with no significant difference between genders (P = 0.73). Table 2 also provides data on acute phase reactants (AFR) measured at the time of diagnosis or admission.
Eighteen patients presented with proteinuria. One patient had nephrotic-level proteinuria (40 mg/m²/hour), which was associated with renal amyloidosis confirmed by kidney biopsy, and this patient was later monitored for chronic kidney failure. Nephritic-level proteinuria (4 - 40 mg/m²/hour) was observed in 17 patients, two of whom underwent rectal biopsies that showed no evidence of amyloidosis. Renal biopsies were performed on two patients, with one revealing HSV nephritis and the other focal segmental glomerulosclerosis (FSGS). Five children exhibited orthostatic proteinuria, and follow-up laboratory tests showed no proteinuria or pathology in other patients.
4.3. Clinical Implications of MEFV Mutations
The predominant mutation observed was heterozygous (n = 146, 48.3%), followed by homozygous (n = 70, 23.2%) and compound heterozygous mutations (n = 60, 19.9%). In 8.6% of the cases, no mutations were detected. The M694V mutation was the most prevalent homozygous mutation, accounting for 71.4% (n = 50) of homozygous cases. Among the compound heterozygous mutations, M694V/E148Q was the most common, occurring in 20.0% of cases, while M680I/V726A had a frequency of 16.7%.
Additionally, out of the 302 patients analyzed, 72 individuals (23.8%) had the heterozygous M694V mutation, while 51 patients (16.9%) had the homozygous M694V mutation. The heterozygous E148Q mutation was identified in 77 patients, representing 25.5% of the study population, while the homozygous E148Q mutation was observed in 7 individuals, making up 2.3% of the total sample.
Table 3 presents the frequency of mutations observed in the 302 patients. The most common MEFV mutations were M694V, E148Q, M680I, and V726A, respectively.
| Genotype | Alleles (n) | Frequency (%) |
|---|---|---|
| M694V | 174 | 42.6 |
| E148Q | 91 | 22.3 |
| M680I | 42 | 10.3 |
| V726A | 38 | 9.3 |
| R761H | 19 | 4.7 |
| M694I | 11 | 2.7 |
| A744S | 10 | 2.5 |
| P369S | 10 | 2.5 |
| R202Q | 4 | 1.0 |
| R408Q | 3 | 0.7 |
| E167D | 2 | 0.5 |
| K695R | 1 | 0.2 |
| S650Y | 1 | 0.2 |
| G687A | 1 | 0.2 |
| L110P | 1 | 0.2 |
| Total | 408 | 100 |
An evaluation was conducted to determine the correlation between mutations and various factors, including gender, age, age at onset of complaints, and clinical and laboratory findings. No statistically significant difference was found between mutation types and gender (P = 0.6).
The assessment of patients' ages at diagnosis and the age at onset of complaints revealed a significant link between the presence of the M694V mutation and both the age at diagnosis and the average age at onset of complaints (P = 0.009, P = 0.001). Patients with the M694V mutation were diagnosed at an earlier age (85 ± 40 months) and experienced earlier onset of symptoms (62 ± 38 months).
An analysis of the relationship between symptoms and mutations showed a significant association between the presence of arthritis and the M694V mutation (P = 0.004). There was also a slight indication of a relationship between chest pain and the M694V mutation (P = 0.065). The presence of arthritis was significantly associated with homozygous mutations (P = 0.001). Additionally, there was a notable association between the M694V mutation and anemia (P = 0.012). Elevated AFR levels were significantly associated with the presence of M694V and E148Q mutations (P < 0.001 and P = 0.02, respectively).
4.4. The Impact of the Mutation Type on Treatment Response
All patients began colchicine treatment upon diagnosis. The typical dosage administered was 0.99 mg/m², but in some cases, it was as high as 1.95 mg/m². After receiving treatment, 43.4% of the patients did not experience any further attacks. Colchicine resistance is defined as experiencing one or more attacks per month over a 3-month period or having persistently elevated C-reactive protein or serum amyloid A levels between attacks. A total of 56.6% of patients experienced at least one attack, and 9.7% had at least three attacks per year. Additionally, 9 patients (3.5%) reported having four or more attacks annually after starting treatment. Colchicine-related adverse effects were observed in only six patients (2.1%) out of the 289 patients. Table 4 presents the various mutation types observed in patients who experienced at least one attack after colchicine treatment.
| Type of Mutation | Attack Positive | Attack Negative | P-Value |
|---|---|---|---|
| Homozygous | 36 (24) | 21 (18.3) | 0.26 |
| Heterozygous | 73(48.7) | 58 (50.4) | 0.77 |
| Compound heterozygous | 28 (18.7) | 27 (23.5) | 0.33 |
| No mutation | 13 (8.7) | 9 (7.8) | 0.8 |
| Total | 150 (100) | 115 (100) | - |
a Values are expressed as No. (%).
E148Q (37.5%) and M694V (31.0%) were the most prevalent mutations identified, with the majority being heterozygous. Among the homozygous mutations, M694V was the most common, accounting for 75.0% of cases. The M680I mutation was observed in 13.9% of cases. The predominant compound heterozygous mutations were M694V/E148Q (21.4%), followed closely by M680I/M694V at 17.9%. No statistically significant difference in mutation type and characteristics was observed between patients with and without attacks (P > 0.05). Due to irregular follow-up visits, treatment response could not be assessed in 37 patients.
Further research was conducted to explore the potential impact of demographic, medical, and genetic factors on colchicine resistance. Initial correlation analysis suggested a possible association between arthritis, arthralgia, and resistance to colchicine. Further analysis revealed additional factors that may serve as predictors of colchicine resistance.
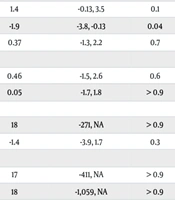
Table 5 presents the results from both univariate and multivariate logistic regression analyses, which aimed to study the impact of demographic and genetic traits on colchicine resistance. The outcome was coded as 0 for resistance and 1 for normal response. In the univariate analysis, arthritis showed a negative association with the outcome (estimate: -2.2, 95% CI: -3.6, -0.80, P = 0.004), and a near-significant association was observed with the homozygous M694V mutation (estimate: -1.4, 95% CI: -2.9, 0.06, P = 0.05). Additionally, males exhibited a positive but nonsignificant association (estimate: 1.4, 95% CI: -0.04, 3.3, p = 0.058). Other traits, including arthralgia and genetic mutations (E148Q, M680I, V726A, R761H), did not demonstrate significant correlations, as indicated by wide confidence intervals and P-values suggesting considerable uncertainty.
In the multivariate analysis, arthritis continued to show a significant negative impact on the outcome after adjusting for potential confounding factors [log (OR): -1.9, 95% CI: -3.8, -0.13, P = 0.038]. However, no statistically significant associations were found for gender [log (OR) for males: 1.4, 95% CI: -0.13, 3.5, P = 0.1], arthralgia [log (OR): 0.37, 95% CI: -1.3, 2.2, P = 0.7], or the genetic mutations (M694V, E148Q, M680I, V726A, R761H) in either heterozygous or homozygous states.
| Variables | N | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Estimate | 95% CI | P | Log (OR) | 95% CI1 | P | |
| Gender | 277 | 0.058 | |||||
| Female | - | - | - | - | |||
| Male | 1.4 | -0.04, 3.3 | 1.4 | -0.13, 3.5 | 0.1 | ||
| Arthritis | 267 | -2.2 | -3.6, -0.80 | 0.004 | -1.9 | -3.8, -0.13 | 0.04 |
| Arthralgia | 267 | -0.74 | -2.1, 0.53 | 0.3 | 0.37 | -1.3, 2.2 | 0.7 |
| M694V | 277 | 0.2 | |||||
| Heterozygous | -0.2 | -1.9, 1.8 | 0.46 | -1.5, 2.6 | 0.6 | ||
| Homozygous | -1.4 | -2.9, 0.06 | 0.05 | -1.7, 1.8 | > 0.9 | ||
| E148Q | 277 | 0.032 | |||||
| Heterozygous | 17 | -93, NA | 18 | -271, NA | > 0.9 | ||
| Homozygous | -1.3 | -3.2, 1.7 | -1.4 | -3.9, 1.7 | 0.3 | ||
| M680I | 277 | 0.3 | |||||
| Heterozygous | 16 | -152, NA | 17 | -411, NA | > 0.9 | ||
| Homozygous | 16 | -481, NA | 18 | -1,059, NA | > 0.9 | ||
| V726A | 276 | 0.2 | |||||
| Heterozygous | 16 | -127, NA | 18 | -291, NA | > 0.9 | ||
| Homozygous | 16 | -2,174, NA | 19 | -5,900, NA | > 0.9 | ||
| R761H | 277 | 0.6 | |||||
| Heterozygous | 15 | -126, NA | 18 | -567, NA | > 0.9 | ||
| Homozygous | 15 | -1,313, NA | 19 | -5,914, NA | > 0.9 | ||
Abbreviations: OR, odds ratio; CI, confidence interval.
5. Discussion
Familial Mediterranean fever, a prevalent inherited inflammatory disease, typically presents with recurring fever and serositis, with a male-to-female ratio of approximately 1.2 - 2:1 (5, 12). In our study, the ratio was 1:1.08.
Given the genetic transmission of FMF, it is likely that relatives will experience this condition more frequently. Our study findings show that 41.1% of our patients had consanguineous parents, while a separate cohort study conducted in 2022 reported a rate of 30.5%. Approximately 28% of the individuals in our study had a family history of FMF, in contrast to the 55.2% reported in the other study (13).
The results of our study revealed that the onset of symptoms and age at diagnosis did not differ by gender. However, published literature indicates a slight bias toward earlier onset in males (14-16).
Consistent with recent studies, we found that abdominal pain (93.7%) was one of the most common symptoms, while fever occurred in 69.0% of cases. In contrast, the occurrence of fever in the published literature exceeds 83% (13, 15-22). The lower occurrence of fever in our study may be due to limitations in data sources or the failure of families to monitor their children's temperature during attacks, instead focusing on associated abdominal pain. The prevalence of other symptoms was in line with what has been reported in the literature (15-22).
Occasionally, headaches may accompany FMF attacks (13). In our patient population, headaches occurred in 13.4% of cases, with noticeable improvement following colchicine treatment. Of the 39 patients who reported headaches, only two were diagnosed with migraines. Although the prevalence of headaches in FMF patients has been reported to reach as high as 46%, most studies report comparable findings to ours (18, 23).
A distinctive sign of FMF, erysipeloid erythema (ELE), can manifest in 3 - 46% of specific cases (5, 17, 23, 24). While 5.5% of our patients had a history of rash, we did not observe any signs of ELE during follow-ups.
Although proteinuria is a significant indicator of amyloidosis, it should not always be assumed that proteinuria confirms the presence of amyloidosis. In our study, we observed amyloidosis in only one case among the patients with proteinuria, while five patients exhibited orthostatic proteinuria. Studies report that the occurrence of amyloidosis can range from 0.4% to 61.9%, with a higher prevalence in the Turkish population. It is also important to consider that a delayed diagnosis of FMF significantly increases the risk of developing amyloidosis (17, 25, 26). Despite the higher occurrence of proteinuria in FMF patients, the considerable issue of amyloidosis, particularly its detrimental effects on the kidneys, must be acknowledged (26).
5.1. Genetic Perspective
Among the 408 mutant alleles in our study, the most frequent mutations were M694V, E148Q, M680I, and V726A, in that order. Studies have consistently demonstrated that the M694V mutation is the most frequently observed in FMF patients (18, 19, 22). Additionally, the E148Q variant was found at a significant frequency of 22.3% in this region.
In Turky, a multicenter study revealed that homozygous M694V (28.1%) is the most common MEFV gene mutation in FMF patients, followed by heterozygous M694V (19.7%). The most common compound heterozygous mutation was M694V/M680I (8.2%). In this study, the E148Q mutation rates were 6.3% for heterozygous, 0.9% for homozygous, and 3.9% for compound heterozygous mutations (19). The E148Q mutation was the most frequently observed heterozygous mutation in our cohort, while the M694V/E148Q mutation was the most common compound heterozygous mutation.
While there may be disagreements concerning the E148Q variant, the diagnosis primarily hinges on clinical criteria. Genetic mutations suggest the presence of the disease, and their absence does not rule out the possibility (24, 27). The E148Q variation in exon 2 was first described as a mutation in 1998 and is considered the second most common alteration causing FMF in non-Ashkenazi Jewish patients (28). However, some argue that E148Q is a functional polymorphism rather than a disease-causing mutation (29). Among patients in Israel, including Jewish, Arab, and Druze individuals, the frequency of this mutation was determined to be 25%. In Türkiye, 20% of Turkish FMF patients were affected, indicating a slightly lower frequency. A report noted that the E148Q mutation was present in 34% of Japanese FMF patients and 17% of Azeri Turk patients living in Iran (21).
None of these studies, however, could entirely rule out the possibility of phenotypic effects in different ethnic groups. Therefore, the role of the E148Q mutation in FMF pathogenesis remains inconclusive. Available data suggests that the presence of E148Q may be linked to a milder form of the disease and a later onset (19, 21, 29).
The occurrence of fever according to mutation types was found to be 75% in homozygous, 69.7% in heterozygous, and 57.8% in compound heterozygous mutations, while it was 73% in those without any mutation. Among individuals with the homozygous M694V mutation, fever and arthritis were observed in 84% and 26% of cases, respectively. A significant disparity in the occurrence of arthritis, fever, and chest pain was noted among patients with the M694V mutation compared to other mutations. Studies have reported that patients with the homozygous M694V genotype tend to have an earlier onset of symptoms and higher frequencies of arthritis and arthralgia (17, 19, 27, 29).
Several studies have examined how MEFV gene mutations influence clinical presentations. In a study conducted in Turkiye, FMF patients with homozygous M694V and heterozygous E148Q mutations were found to have significantly higher rates of chest pain (17, 30, 31). In our study, 26.6% of patients had anemia. Anemia and elevated AFR were significantly associated with the M694V mutation, a finding that aligns with reports in the literature (29, 32).
5.2. Colchicine Response
Our patients experienced a significant decrease in complaints after receiving colchicine treatment. Despite occasional side effects, colchicine remains the first-line treatment because it is both safe and effective, while also being affordable. Only 2.1% of our patients experienced side effects associated with colchicine.
In a study involving 153 Jewish children diagnosed with FMF, 14.4% experienced diarrhea, and 11.8% had mild, transient transaminase elevation. No significant adverse effects were reported, and all patients exhibited a favorable response to colchicine therapy, with less than two attacks per year (33, 34). Colchicine is considered a safe option for treating FMF, despite potential ethnic differences in adverse reactions.
None of our patients showed colchicine resistance during the study period. Previous research indicates that 2.8% of patients did not respond to colchicine; however, more recent data suggests that the percentage of nonresponsive patients has risen to approximately 4.2% (19, 35). Moreover, several studies in the literature highlight the growing prevalence of colchicine resistance and underscore the clinical implications of alternative treatments such as anakinra (36). A subset of patients (9.7%) experienced more than three attacks per year.
Univariate analysis indicates that patients with arthralgia are more prone to developing resistance to colchicine. Additionally, individuals with a homozygous M694V mutation may experience more favorable outcomes compared to those with heterozygous mutations, warranting further discussion. The results of this study suggest that while certain factors, such as arthritis, have a clear and significant effect on colchicine resistance, other variables like gender and specific genetic mutations do not play an independent role in determining outcomes when controlling for other variables.
Although several studies have been conducted, no distinction in colchicine resistance has been observed across various mutation types (33-35). However, there is compelling evidence in the literature suggesting that individuals with a homozygous M694V mutation may not respond adequately to colchicine. According to Lidar et al., incomplete responses are inferred from the increased dose of colchicine required, its adverse effects, and the higher frequency of attacks per month in homozygous M694V patients (37).
Evidence also indicates that individuals homozygous for M694V are more likely to develop the disease at a younger age and experience a higher incidence of arthritis and arthralgia (17, 19). Our findings closely align with these observations. According to Cekin et al., the homozygous M694V mutation is significantly associated with abdominal pain and fever compared to other mutations in terms of genotype-phenotype relationships. However, there were no distinguishable symptoms between individuals with heterozygous and compound heterozygous mutations (38).
This study has several limitations that should be considered. The retrospective design, which relied on medical records, presents potential biases, including selection bias. Since data collection depended on pre-existing medical records, it is possible that certain variables may have been incomplete or absent, potentially affecting the accuracy and comprehensiveness of the analysis. Larger prospective studies are recommended to address these limitations and further validate our findings.
5.3. Conclusions
Our study underscores the significant clinical heterogeneity present in patients diagnosed with FMF. The homozygous M694V mutation was associated with a more severe disease course, marked by an earlier onset and a higher prevalence of symptoms such as fever, arthritis, and anemia. These findings highlight the crucial correlation between genotype and phenotype in FMF. While the M694V, E148Q, and M680I mutations were commonly observed in the southeastern region of Türkiye, further studies exploring geographic and population-based patterns are needed to fully elucidate the mutational landscape of FMF within the country.
Our analysis also reveals a greater likelihood of colchicine resistance among patients experiencing arthritis. Nonetheless, the results strongly support the ongoing efficacy and cost-effectiveness of colchicine as a key treatment for FMF, despite the variability in clinical outcomes. This reinforces the importance of early diagnosis and adherence to a consistent treatment regimen to manage the disease effectively.