1. Background
Cerebral palsy (CP) is a childhood condition that causes permanent motor disabilities. Depending on the type and severity of CP, children may experience neuromuscular problems such as spasms, contractures, lack of coordination, and weakness. These issues can lead to impairments in motor function and daily activities (1). Hand function is an important aspect of motor function, involving tasks such as reaching for a target, grasping, manipulating, and releasing objects. Children with CP need to coordinate their movement sequences and visual perception skills to perform these tasks effectively (2). However, upper limb function is often limited in children with CP due to changes in muscle tone, contractures of upper limb joints, and a restricted range of joint motion. These limitations can cause difficulties in play and self-care activities, increasing the dependence of children with CP in their daily routines (2-4).
The goal of hand function rehabilitation is to prevent further deformities, enhance manual abilities, increase independence, and improve the overall quality of life for individuals (4). Various rehabilitation treatments are available to improve upper limb function in children with CP, including bimanual task training, constraint-induced movement therapy, context-focused therapy, goal-directed training/functional task training, and transcranial direct current stimulation (tDCS). Transcranial direct current stimulation is a non-invasive brain stimulation technique that applies a low-intensity electrical current directly to specific areas of the brain via electrodes placed on the scalp. It can modulate the resting potential of the neuronal membrane, thereby altering cortical excitability (5). The effectiveness of tDCS depends on the current concentration, which is determined by the strength of the electric field and the size and power of the electrodes. It has been proven that higher current densities lead to stronger stimulation effects (6). Additionally, when tDCS is used repetitively, it can induce long-term changes in cortical function that may enhance functions impaired in musculoskeletal and neurological disorders. Thus, tDCS can modulate brain waves by delivering an electric current at a specific frequency, and if applied over a prolonged period, it can also induce neuroplasticity effects (7-9).
Studies on the use of tDCS for children with CP are limited and inconsistent compared to those conducted on stroke patients. While there have been studies examining the effect of tDCS on balance, gait, and lower limb function in children with CP, research on its impact on upper limb function is lacking (10-12). Previous studies have primarily focused on stimulating the lesioned hemisphere, whereas this study aimed to inhibit the non-lesioned hemisphere. Since rehabilitation treatments for hand function in children with CP can be slow and time-consuming, exploring auxiliary techniques may be beneficial in achieving faster improvements in upper limb function (5, 13).
Transcranial direct current stimulation can be applied in two ways: Stimulating the lesioned hemisphere or inhibiting the non-lesioned hemisphere. This technique can alter the activity and functional connectivity of brain networks in both hemispheres by affecting biological tissues and modulating cell membrane potential. According to the literature, only a limited number of articles have explored the technique of inhibiting the non-lesioned hemisphere, and these studies have typically involved immediate effects of tDCS and a small number of intervention sessions. Therefore, it is necessary to further investigate the effect of this technique, specifically in the form of inhibiting the non-lesioned hemisphere, with more intervention sessions in children with CP.
2. Objectives
This study aimed to investigate the impact of tDCS, in combination with routine occupational therapy exercises, on upper limb function in children with CP.
3. Methods
3.1. Participants
Fifty hemiplegic CP patients diagnosed by a neurologist were selected for a double-blind clinical trial from those who had referred to Karaj clinics. The patients were chosen using non-probability sampling. The study included children aged 5 - 10 years who had been diagnosed with unilateral CP, exhibited difficulty in gross manual dexterity, and had no unstable medical conditions such as epilepsy or any disease that increases brain activity. All participants had mild spasticity based on the Modified Ashworth Scale and were classified as Level I or II on the gross motor function classification system (GMFCS), indicating that they could walk independently or with orthopedic shoes. Patients with metal implants near the electrode site or those receiving other auxiliary treatments, such as Botox injections in the upper limb during the intervention period, were excluded from the study. This study was approved by the Ethical Committee of Shahid Beheshti University of Medical Sciences under the code IR.SBMU.RETECH.REC.1402.096 and was registered in the clinical trial registry with the code IRCT20220725055552N1.
A pilot study was conducted on two groups, each consisting of six CP children, to determine the sample size. Based on the mean and standard deviation of the gross manual dexterity variable in each group, it was determined that at least 22 participants would be needed. Therefore, the present study was conducted with 50 participants, divided into two groups of 25 CP children each.
An independent researcher performed the block randomization method using a computer-generated random number list to assign participants to real tDCS and sham tDCS groups, with an allocation ratio of 1:1, using random block sizes of two. Additionally, the allocation sequence was concealed from the researcher enrolling and evaluating participants.
3.2. Data Gathering Tools
3.2.1. The Transcranial Direct Current Stimulation Device
The ActivaDose tDCS device from Active Tek Company, United States, was used in this research. It is capable of increasing the current intensity up to 5 milliamps. For this study, the intensity was set to 1.5 milliamps. The device features an LCD screen and adjustment buttons, two anode electrodes (stimulating) and two cathode electrodes (inhibitory), two square rubber sheets to hold the head electrodes, two sponge pads, and two bands to secure the pads in place on the head.
3.2.2. Fugl-Meyer Test
To measure motor function with high inter-rater and intra-rater reliability, the Fugl-Meyer test was used in this study (14). The test includes 33 items for the upper limb and 17 for the lower limb, with scoring based on direct observation of performance. The scale uses a 3-point ordinal system where 0 indicates an inability to perform, 1 indicates partial performance, and 2 indicates full performance. The total score is the sum of all items, with a maximum of 66 for the upper limb and 34 for the lower limb. Each task should be completed within a reasonable time frame, with a 20-second cutoff per attempt and a maximum of 3 attempts per test item, based on experience.
3.2.3. Box and Block Test
In this study, the Box and Block Test was used to measure gross manual dexterity with the use of an upper limb prosthetic device. It is a valid and reliable test applicable to various populations, including individuals with CP (15). The standardized equipment consists of a wooden box measuring 53.7 cm × 25.4 cm × 8.5 cm, with a partition dividing it into two compartments of 25.4 cm each, and 150 wooden cubes, each measuring 2.5 cm. Participants are scored based on the number of blocks they move from one compartment to the other within 60 seconds.
3.2.4. Bruininks Oseretsky Test
The Bruininks-Oseretsky test is a reliable and valid assessment tool used to measure fine and gross motor skills in children across four motor areas: Fine manual control, manual coordination, body coordination, and strength and agility. It consists of eight sub-tests and is available in a complete form with 53 items or a short form with 14 items, which was used in this study. The short form has been shown to have high validity and reliability in school-aged children (16, 17).
3.3. Procedure
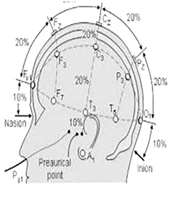
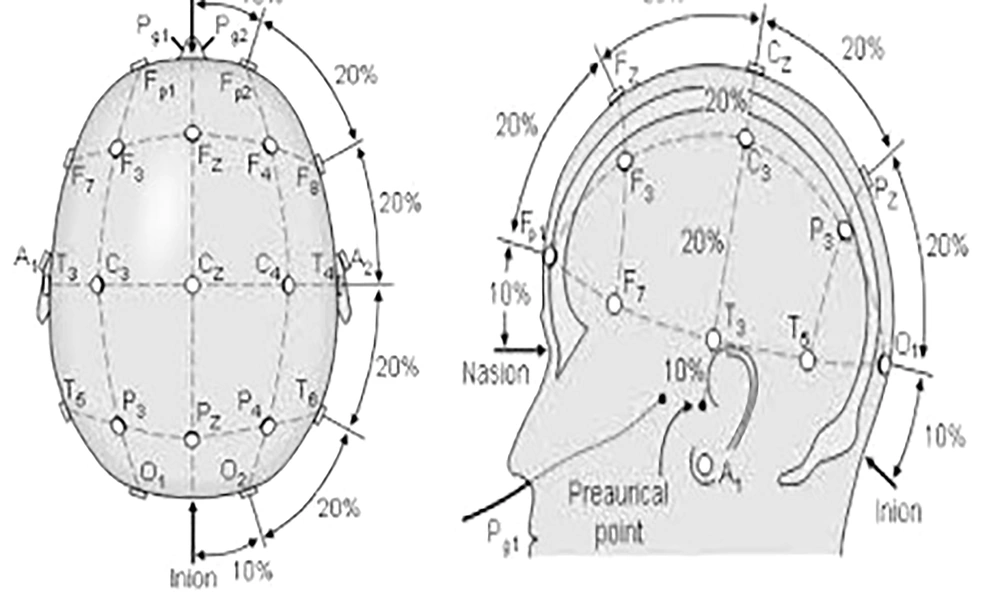
The study began with an explanation of the procedures and potential complications to the families. After obtaining consent, the participants were randomly assigned to either the experimental or control group. The evaluator conducted the Fugl-Meyer, Box and Block, and Bruininks-Oseretsky tests on all children before the intervention sessions began. For the tDCS intervention in the experimental group, the child sat on a chair, and the electrodes were connected to the device. The stimulation points on the subjects' heads were identified based on the international 10 - 20 system (18). Measurements were taken from the middle of the nasal bridge to the middle and end of the occiput, and the Fz, Cz, Pz, and the midpoint between O1 and O2 on the occipital cortex were marked. A pad with the anode electrode was fixed in the O1 region and a pad with the cathode electrode in the O2 region using a strap tape, and the device was adjusted (Figure 1). The experimental group received stimulation for 20 minutes in the primary motor area of the non-lesioned hemisphere, with a current intensity of 1.5 milliamps (cathode) and in the supraorbital area (anode).
After the tDCS session, routine occupational therapy exercises were conducted to improve hand function, such as reach and grasp exercises, releasing objects with one hand and two hands through activities, and improving object manipulation skills with games. These exercises included threading beads, moving a ball from a height, throwing a ball with the involved hand, separating small objects, and strengthening wrist muscles. The occupational therapy sessions lasted for 45 minutes.
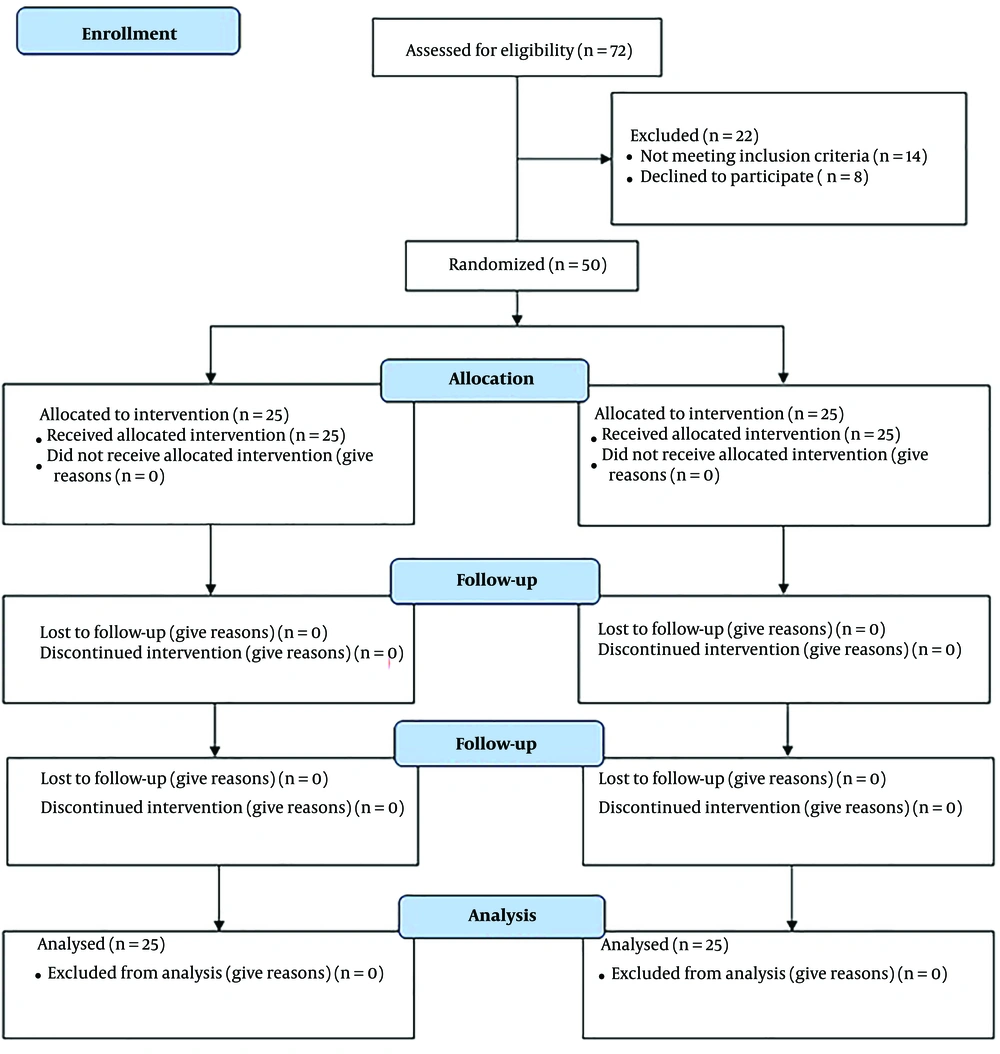
In the study, the control group underwent a 20-minute intervention where the current was cut off after 30 seconds. The participants were unaware of the current interruption during the intervention, and occupational therapy exercises were performed for 45 minutes. The intervention was conducted for four weeks, with five sessions per week. After the treatment sessions, the participants were evaluated using the Fugl-Meyer, Box and Block, and Bruininks-Oseretsky tests. Since all therapeutic interventions were conducted face-to-face under the supervision of the occupational therapist (main researcher), all subjects participated in every therapeutic session and completed the study. The steps of the study are outlined in the CONSORT flowchart (Figure 2).
The evaluator, participants, and statistician were all blinded to the group assignments. Additionally, participants were unaware of the type of stimulation they received (sham or real tDCS). While the therapeutic interventions were administered by the main investigator, the outcome measures were evaluated by another person.
3.4. Statistical Analysis
The statistical analysis of the data was performed using SPSS 21 software with a significance level of P < 0.05. The normality of the data was assessed using the Shapiro-Wilk test. For intra-group comparisons, a paired t-test was used for data with a normal distribution, and the Wilcoxon test was used for data without a normal distribution. For inter-group comparisons, pre- and post-intervention, an independent t-test was used for data with a normal distribution, and the Mann-Whitney test was used for data without a normal distribution. Additionally, the effect sizes of each variable were calculated based on Cohen's D, with values higher than 70 percent indicating a high impact of the intervention.
4. Results
A total of 50 CP patients were evaluated and analyzed in the study, with 25 in each group. Of the participants, 56% were boys, 56% were aged between 7 - 10 years old, and 44% were aged between 5 - 7 years old. Additionally, 52% of the participants had left-sided hemiplegia. Table 1 displays the mean, standard deviation, and median of the variables.
| Variables | Experimental Group (n = 25) | Control Group (n = 25) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Mean Difference | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Mean Difference | Pre-treatment | Post-treatment | |
| Gross manual dexterity | 66.36 ± 8.45 | 101.20 ± 9.22 | 34.84 | 70 | 100 | 63.36 ± 15.43 | 73.36 ± 15.42 | 10 | 65 | 74 |
| Manual coordination | 25.56 ± 1.58 | 37.68 ± 1.63 | 12.12 | 26 | 38 | 22.84 ± 3.69 | 29.68 ± 3.57 | 6.84 | 24 | 31 |
| Fine manual control | 27.32 ± 3.73 | 39.80 ± 1.80 | 12.48 | 28 | 41 | 26.52 ± 4.44 | 34.36 ± 5.01 | 7.84 | 27 | 36 |
| Body coordination | 25.56 ± 3.83 | 38.08 ± 2.74 | 12.52 | 27 | 40 | 24.12 ± 4.20 | 31.96 ± 4.40 | 7.84 | 25 | 32 |
| Strength and agility | 29.72 ± 3.29 | 43.04 ± 2.39 | 13.32 | 30 | 44 | 26.16 ± 5.11 | 33.72 ± 4.81 | 7.56 | 27 | 35 |
| Motor function | 35 ± 4.99 | 51.72 ± 4.12 | 16.72 | 35 | 53 | 34.76 ± 6.85 | 40.88 ± 6.41 | 6.12 | 35 | 42 |
a Values are expressed as mean ± SD or median.
b Mean difference: Difference between mean post treatment and mean pretreatment.
There was no significant difference in the mean of all variables related to upper limb function before the intervention between the two groups (P > 0.05).
The outcome measures showed a significant improvement (increase) in both groups after the 4-week intervention compared to pre-intervention (P < 0.001, Table 2). However, the mean difference in the experimental group was higher than that of the control group, indicating a significant improvement in all outcome measures in the experimental group compared to the control group in the post-treatment results (Table 2). Based on the effect size values reported in Table 2, the tDCS intervention had a high impact (effect size > 0.70) on all research variables.
Moreover, no significant side effects were observed during or after the use of tDCS in this study.
| Variables | Intragroup Pre-post Comparison in Experimental Group (n = 25) | Intragroup Pre-post Comparison in Control Group (n = 25) | Intergroup Comparison After Intervention | Effect size (Cohen's D) |
|---|---|---|---|---|
| Gross manual dexterity | < 0.001 | < 0.001 | < 0.001 | 2.19 |
| Manual coordination | < 0.001 | < 0.001 a | < 0.001 a | 2.88 |
| Fine manual control | < 0.001 | < 0.001 a | < 0.001 a | 1.44 |
| Body coordination | < 0.001 | < 0.001 a | < 0.001 a | 1.66 |
| Strength and agility | < 0.001 | < 0.001 a | < 0.001 a | 2.45 |
| Motor function | < 0.001 | < 0.001 a | < 0.001 a | 2.01 |
a Non-parametric test.
5. Discussion
During the rehabilitation process, neuromodulation techniques are used to enhance the effectiveness of local synapses and modify maladaptive plasticity patterns that may occur following a cerebral cortex lesion. Transcranial direct current stimulation is a non-invasive treatment that stimulates or inhibits the primary motor cortex by delivering a low-intensity direct current through surface electrodes. This neuromodulation tool can alter the activity and functional connectivity of brain networks in both hemispheres by affecting biological tissues and modulating cell membrane potential. When a continuous weak electric current is applied to the scalp using the anode electrode, it increases the excitability of the motor cortex, while the cathode electrode reduces motor cortex stimulation (19, 20).
The results of this study demonstrated that both groups showed improvement in all upper limb function outcomes after the intervention. However, the group that received tDCS exhibited more significant improvement than the control group, which only received routine occupational therapy.
The findings of the current study align with previous research that highlighted the positive effects of tDCS intervention on upper limb function in neurological diseases such as stroke or CP. However, it is important to note some differences in the treatment protocol of this study compared to previous ones. In earlier studies on upper limb function in children with CP, the tDCS treatment protocol focused on stimulating the lesioned hemisphere (19-21). In contrast, the present study inhibited the non-lesioned hemisphere, allowing for increased activity in the lesioned hemisphere. The improvement in past studies was often less long-lasting, potentially due to the smaller number of treatment sessions (19), or immediate improvement was reported without long-term follow-up (21). In Moura et al.'s study (20), as in other previous studies (22, 23), overall hand function was assessed using tests like the quality of upper extremity skills. However, in the present study, upper limb function and abilities were examined in greater detail.
The present study followed a treatment protocol consistent with Rich et al.'s study, which involved inhibiting the non-lesioned hemisphere with a current of 1.5 milliamps for twenty minutes (22). However, there were differences in terms of the combination of interventions, sample size, age, and the number of treatment sessions. In Rich et al.'s study, bimanual training was conducted for ten sessions on only eight CP children aged seven to twenty-one after inhibiting the non-lesioned hemisphere (22). In contrast, the present study involved exercises such as throwing and moving a ball, strengthening wrist muscles, threading beads, and separating small objects for twenty sessions following tDCS in twenty-five children with CP aged five to ten years. Another twenty-five children with CP performed these occupational therapy exercises with sham tDCS as the control group, with significant improvement in measured outcomes in both groups.
Gillik et al.'s study involved ten consecutive weekday sessions of tDCS applied to the non-lesioned hemisphere (20 minutes) concurrently with constraint-induced therapy (120 minutes), and both groups showed significant improvement in hand function after the intervention. While complications such as headaches and itchiness were commonly reported, no significant effect of tDCS was observed in that study (23). This study aligns with theirs in terms of the significant improvement in hand function and the inhibition protocol, though this study used a 1.5 milliamp current, while Gillik et al.'s study (23) used 0.7 milliamps. Additionally, no significant side effects were observed in this study. Systematic reviews also reported no serious adverse events during tDCS in pediatric populations, with tolerability improving over time and side-effect frequency decreasing (24, 25). The slight differences in results may be due to the auxiliary techniques used in addition to the tDCS technique, as constraint-induced therapy was used in their study, while a different therapy technique was used in the present study. Moreover, the evaluation methods for upper limb function may also explain the differences between this study and the previous two. In earlier studies, hand function was assessed more generally, while the present study examined it in greater detail.
As mentioned, tDCS can be applied by stimulating the lesioned hemisphere or inhibiting the non-lesioned hemisphere. However, only a limited number of articles have been published on this subject in the last twenty years, and out of this small number, only two studies have specifically examined the effect of inhibiting the non-lesioned hemisphere (22, 23). These previous studies have been criticized for their small sample sizes, the immediate effect of tDCS, or the limited number of intervention sessions. To our knowledge, the present study is the only one to investigate the effect of tDCS by inhibiting the non-lesioned hemisphere in a larger sample of CP children. Furthermore, this study assessed upper limb function in detail using tDCS. Based on the results of this and previous studies, we can conclude that tDCS, combined with other occupational therapy exercises, has a positive effect on improving upper limb function and can be used as a non-pharmacological rehabilitation method.
The present study had some limitations that should be considered when interpreting the results. One limitation was that only children aged five to ten years were evaluated, whereas previous studies included participants with a wider age range. Therefore, generalizing the results of this study to other age groups should be done with caution. Another limitation was that the type of hemiplegia (left or right) was not compared. Future studies could investigate the effect of age on the improvement of upper limb function in children with CP while conducting the intervention. Additionally, types of CP (quadriplegia and diplegia) were not included in our study. Finally, the lack of a follow-up period after the treatment phase meant that the lasting effect of tDCS could not be determined. Therefore, evaluating the long-term effects of the interventions could further clarify the differences between the two treatments.
5.1. Conclusions
This study demonstrated that while both therapeutic interventions improved upper limb function in children with unilateral CP, the combined intervention of tDCS and occupational therapy was more effective in improving outcome measures compared to routine occupational therapy alone. These results have clinical implications and suggest that using these modalities in a rehabilitation program for children with CP is recommended.
5.2. Clinical Implications
- Transcranial direct current stimulation is a non-invasive treatment that stimulates or inhibits the primary motor cortex by applying a low-intensity direct current through surface electrodes.
- The combined intervention of tDCS and occupational therapy is recommended for achieving better results in improving upper limb function in children with CP.