1. Background
Neonatal respiratory distress syndrome (RDS), also known as hyaline membrane disease (HMD), occurs due to a deficiency of pulmonary surfactant (PS), leading to rapid onset, fast progression, and poor prognosis of the disease (1). Respiratory distress syndrome poses a high risk of morbidity in premature infants, characterized by symptoms such as progressive respiratory distress and cyanosis shortly after birth, making it necessary for such infants to receive early and effective treatment to improve survival rates (2). Respiratory distress syndrome is a common complication in premature infants and a significant risk factor for premature infant mortality. In recent years, with advancements in clinical medicine, mechanical ventilation and PS replacement therapy have been increasingly applied in the treatment of RDS, leading to a significant decrease in mortality rates associated with the disease (3). However, global survey data indicate that RDS accounts for approximately 40% of deaths in children under 5 years old (4). Specifically, premature infants have underdeveloped lungs and a congenital PS deficiency, leading to severe respiratory failure symptoms. Meanwhile, poor disease control can result in long-term respiratory and neurological sequelae, affecting the healthy development of the infants and increasing the risk of premature infant mortality. In contrast, late preterm infants refer to those with a gestational age of 34 - 36+6 weeks, accounting for about 72% of premature infants. Studies have shown that, compared to full-term infants, late preterm infants experience significantly higher mortality rates and short- and long-term complications, among which RDS is the most prevalent, with an incidence of around 8.3% in late preterm infants (5). The occurrence of RDS in premature infants is due to PS deficiency, and existing clinical evidence indicates that the risk factors for the disease include gestational age and birth weight.
2. Objectives
However, due to the complex etiology of RDS, in-depth research is needed to explore other risk factors beyond the known ones, thus facilitating early detection and treatment of the disease. This study is designed to analyze the perinatal high-risk factors for late preterm infants with RDS treated at our hospital, with the aim of facilitating early diagnosis and treatment of the disease. The findings are reported as follows.
3. Methods
3.1. Demographic Characteristics
This was a case-control study. One hundred and thirty-eight late preterm infants admitted to our hospital from January 2022 to December 2023 were selected and divided into the observation group (n = 36) and the control group (n = 102) based on the occurrence of RDS.
3.1.1. Inclusion Criteria
(1) Patients who met the diagnostic criteria for neonatal RDS, including clinical manifestations of accelerated breathing, respiratory rate > 60 breaths/min, groaning, and three concave signs. Arterial blood gas analysis suggested hypoxemia and hypercapnia. Radiographs indicated poor lung aeration with fine granular shadows and air bronchogram.
(2) Patients who were admitted after delivery.
3.1.2. Exclusion Criteria
(1) Patients with congenital inherited metabolic diseases.
(2) Patients with combined bronchopulmonary dysplasia, pneumonia, or asphyxia.
(3) Patients with intracranial hemorrhage or other diseases.
3.2. Methodology
The clinical data of the two groups were collected, and the high-risk factors were analyzed based on the obtained data, followed by a statistical analysis. The possible bias of retrospective data in this study is mainly due to various factors in the process of data collection and processing, including the span of the data years and the subjective tendencies of the researchers. To reduce measurement bias related to the data year span, the data collected from January 2022 to December 2023 has been determined to be derived from standardized test methods and instruments. Additionally, there is no conflict of interest between the researcher and the study, and the study has been approved by the ethics committee, which helps reduce researcher bias.
3.3. Statistical Methods
The data obtained in this study were entered into SPSS 21.0 software for calculation, with measurement data expressed as percentages (%), and the chi-square (χ2) test was used. Multivariate analysis was performed using logistic regression analysis. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Comparison of General Data Between the Two Groups
There was no significant difference in gender, gestational age, and weight between the two groups (P > 0.05), indicating excellent comparability (Table 1).
| Groups | No. | Gender | Gestational Age (wk) | Weigh (g) | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Observation | 36 | 20 (55.56) | 16 (44.44) | 35.08 ± 0.91 | 2334.78 ± 405.61 |
| Control | 102 | 55 (53.92) | 47 (46.08) | 35.21 ± 0.85 | 2341.05 ± 401.96 |
| χ2/t | - | 0.029 | 0.774 | 0.080 | |
| P | - | 0.866 | 0.440 | 0.936 | |
Comparison of Demographic Characteristics Between the Two Groups a
4.2. Comparison of Perinatal High-Risk Factors in Late Preterm Infants
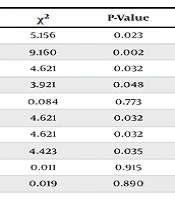
Univariate analysis of perinatal high-risk factors in late preterm infants revealed significant differences between the two groups in male sex, elective cesarean section, amniotic fluid aspiration, premature rupture of membranes (PROM), intrauterine hypoxia, and pregnancy complications (P < 0.05) (Table 2).
| Factors | Observation (n = 36) | Control (n = 102) | χ2 | P-Value |
|---|---|---|---|---|
| Male | 22 (61.11) | 40 (39.22) | 5.156 | 0.023 |
| Elective cesarean section | 30 (83.33) | 56 (54.90) | 9.160 | 0.002 |
| Amniotic fluid aspiration | 7 (19.44) | 7 (6.86) | 4.621 | 0.032 |
| PROM | 2 (5.56) | 20 (19.61) | 3.921 | 0.048 |
| Meconium-stained amniotic fluid | 1 (2.78) | 2 (1.96) | 0.084 | 0.773 |
| Intrauterine hypoxia | 7 (19.44) | 7 (6.86) | 4.621 | 0.032 |
| Gestational diabetes | 7 (19.44) | 7 (6.86) | 4.621 | 0.032 |
| Gestational hypertension | 8 (22.22) | 9 (8.82) | 4.423 | 0.035 |
| Multiple pregnancy | 12 (33.33) | 35 (34.31) | 0.011 | 0.915 |
| Maternal age over 35 years | 6 (16.67) | 16 (15.69) | 0.019 | 0.890 |
Comparison of Perinatal High Risk Factors in Late Preterm Infants a
4.3. Multivariate Analysis of Factors Affecting the Occurrence of RDS in Late Preterm Infants
The multivariate logistic regression analysis of the univariate factors with statistical significance found that the high-risk factors for RDS in late preterm infants included all factors except for intrauterine hypoxia (P < 0.05) (Table 3).
| Factors | β | SE (β) | Waldχ2 | P-Value | Exp (β) | 95%CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Male | 1.120 | 0.278 | 10.452 | < 0.001 | 0.399 | 0.228 | 0.698 |
| Elective cesarean section | 2.361 | 0.348 | 46.851 | < 0.001 | 10.568 | 5.394 | 20.748 |
| Gestational diabetes | 0.625 | 0.103 | 20.421 | < 0.001 | 1.865 | 1.552 | 2.490 |
| Gestational hypertension | 0.637 | 0.108 | 18.732 | < 0.001 | 1.874 | 1.546 | 2.482 |
| Amniotic fluid aspiration | 0.621 | 0.251 | 2.997 | 0.040 | 1.678 | 1.208 | 2.068 |
| PROM | -10.82 | 0.504 | 4.547 | 0.032 | 0.338 | 0.131 | 0.922 |
| Intrauterine hypoxia | -132 | 0.411 | 0.099 | 0.748 | 1.485 | 0.138 | 0.865 |
Multivariate Analysis of Factors Affecting the Occurrence of Respiratory Distress Syndrome in Late Preterm Infants
5. Discussion
Compared to full-term infants, late preterm infants are immature in development and prone to neonatal complications, among which RDS is a common critical illness in newborns. Respiratory distress syndrome is characterized by rapid onset and progression, with a mortality rate of up to 50% in newborns with acute RDS due to a lack of prompt and effective treatment (6, 7). The occurrence of RDS is closely associated with a deficiency of PS, a phospholipid-protein complex synthesized and secreted by type II alveolar epithelial cells. PS covers the surface of the alveoli and significantly reduces alveolar surface tension, thereby playing an essential role in preventing alveolar collapse at the end of expiration and helping maintain functional residual capacity (FRC) (8).
During pregnancy, PS production begins around 18 - 20 weeks of gestation and increases with the duration of pregnancy, with a significant surge around 35 - 36 weeks of gestation, reaching the level of lung maturity in a short period of time (9). Therefore, a younger gestational age indicates a lower PS content, increased alveolar surface tension, decreased FRC at end-expiration, and a higher risk of alveolar collapse. Additionally, existing research demonstrates that premature infants with younger gestational ages and lower birth weights experience a higher risk of RDS.
Newborns require an adequate amount of PS after birth to reduce pulmonary tension, increase pulmonary compliance, stabilize alveolar volume, prevent alveolar collapse, maintain normal fluid pressure between alveoli and capillaries, and reduce the risk of pulmonary edema. Adequate PS also acts on precapillary vessels to reduce tension, significantly increasing pulmonary ventilation and creating favorable conditions for pulmonary artery dilation (10, 11). During the first breath after birth, newborns need to overcome various resistances such as fluid pressure in the trachea and surface tension. This indicates that lower birth weight means a higher risk of RDS in premature infants. Both gestational age and birth weight are indicators of infant maturity, with increased maturity leading to a reduced risk of RDS (12).
Previous studies have mostly focused on indicating that the occurrence of RDS is closely related to gestational age and birth weight, and recent studies have shown that it is also associated with various other factors. Common risk factors include gestational diabetes, multiple pregnancies, infant gender, PROM, cesarean section, intrauterine distress, and perinatal asphyxia (13). Compared to full-term infants, late preterm infants are immature in development, with a gestational age between 34 - 36 weeks. Their lung development is in the terminal sac stage, transitioning to the alveolar stage, which poses a higher risk of PS deficiency and increases the risk of respiratory system complications.
This study revealed that in late preterm infants, there are more males in the observation group than in the control group, which is consistent with relevant domestic literature, indicating that male sex is a risk factor for RDS during the perinatal period in late preterm infants. This is because male premature infants have decreased lung function, underdeveloped immune organs such as the thymus, and weaker resistance, making them more susceptible to RDS (14). Meanwhile, among the late preterm infants selected for this study, no significant difference was observed in gestational age and weight between the two groups, as both groups consisted of late preterm infants with a small range of gestational ages, resulting in minimal differences in gestational age and weight.
This study suggested a higher proportion of amniotic fluid aspiration in the observation group than in the control group among late preterm infants, and logistic analysis identified amniotic fluid aspiration as a risk factor for perinatal RDS in late preterm infants. Specifically, other substances in the amniotic fluid (such as fetal fat, shed cells, microorganisms, etc.) can cause inflammation and increase alveolar permeability when inhaled. Meanwhile, damage to type II alveolar epithelial cells in premature infants and inadequate production of PS reduces pulmonary compliance, increases elastic resistance, and severely impairs lung function, ultimately increasing the risk of RDS (15). Therefore, for late preterm infants, it is necessary to quickly remove foreign objects from the airway after delivery and minimize amniotic fluid aspiration to effectively prevent RDS.
Currently, there is a lack of unified consensus on whether PROM is a risk factor for RDS in premature infants. Some studies suggest that PROM is a risk factor for RDS in premature infants due to the increased risk of secondary infection after PROM, leading to an increased risk of RDS after fetal delivery (16, 17). However, some scholars believe that PROM is a protective factor for RDS due to a change in hormone levels in the fetal body after its occurrence, resulting in early delivery, which can effectively prevent infection and reduce the occurrence of RDS.
Additionally, the body experiences a stress response after PROM, leading to an increase in endogenous cortisol, which effectively aids in lung maturity, thereby reducing the risk of RDS. This study indicated a lower proportion of PROM in the observation group than in the control group, and a logistic analysis suggested that it is a protective factor for RDS in late preterm infants rather than a risk factor. However, in-depth clinical investigations are needed to explore the specific mechanisms.
In the meantime, this study revealed a significantly higher proportion of elective cesarean sections in the observation group than in the control group, and a logistic analysis identified this as a high-risk factor for RDS in late preterm infants. The reason is that there is a significant increase in the secretion of fetal steroids and catecholamines during natural childbirth, which greatly facilitates lung maturity. In contrast, for fetuses born by cesarean section, the assistance in lung maturity is minimal due to no significant change in hormone levels in the body. Additionally, due to the lack of extrusion through the birth canal, there is a significant decrease in the formation of negative intrathoracic pressure compared to that in infants born through natural delivery, making it difficult to effectively expel airway and lung fluids. Furthermore, late preterm infants born by cesarean section demonstrate relatively lower levels of endogenous glucocorticoid, which negatively impacts PS production (18).
Additionally, this study also suggested a higher proportion of gestational diabetes and gestational hypertension in the observation group than in the control group, and the logistic analysis identified pregnancy complications as high-risk factors for RDS in late preterm infants. The analysis revealed that pregnant women with pregnancy complications are more likely to choose cesarean section due to their own factors, leading to a higher risk of delivery complications, thereby increasing the risk of RDS. In cases of gestational diabetes during pregnancy, the fetus often experiences hyperglycemia and hyperinsulinemia, which exhibit adverse effects on the maturation of type II lung cells and inhibit PS production, resulting in an increased risk of RDS (19).
5.1. Limitation
There are still some shortcomings in this study. This study was a retrospective descriptive analysis, and the number of subjects included was limited, so the conclusions drawn may not be highly convincing. Additionally, we only analyzed and discussed the cases from our hospital, which may not be fully representative. We look forward to a multi-center study in the future to reach more comprehensive conclusions. Further intervention trials are needed to confirm these results.
5.2. Conclusions
In conclusion, the occurrence of perinatal RDS in late preterm infants is closely related to numerous factors, with the maturity of premature infants being directly associated with the occurrence of RDS. In addition, factors such as perinatal pregnancy complications, amniotic fluid aspiration, gender, and others are also closely related to the occurrence of RDS. Therefore, it is of great significance to prevent RDS through the following clinical measures: Carefully monitoring premature infants, closely observing the progress of labor, promptly identifying high-risk factors, implementing targeted interventions, and choosing the appropriate time to terminate the pregnancy.