1. Background
Caffeine citrate, one of the most frequently prescribed medications in the neonatal intensive care unit (NICU), is the primary drug of choice for treating apnea of prematurity (AOP). The effects of caffeine citrate have been mainly described based on clinical outcome parameters. In addition to decreasing AOP, caffeine citrate decreases extubation failure (1-3) and bronchopulmonary dysplasia, resulting in better neurodevelopmental outcomes (4-6). The mechanism of action of caffeine citrate includes stimulation of the respiratory center in the brainstem, increasing sensitivity to carbon dioxide, and enhancing muscular tone and contractility of the respiratory muscles and diaphragm (7).
The direct effect of caffeine citrate on the activation of diaphragmatic motion has not been extensively researched. Only a few studies have reported on the changes in diaphragmatic activity after caffeine citrate administration using transcutaneous diaphragmatic electromyography (dEMG) (8-10). However, due to the inconsistency of transcutaneous dEMG amplitudes due to factors such as electrode attachment sites, patient skin status, hardware and/or software types (10, 11), the application of transcutaneous dEMG in preterm infants for whom caffeine citrate administration may be the most beneficial and thus approved for use, is still limited.
Neurally adjusted ventilatory assist (NAVA), which uses the electrical activity of the diaphragm (Edi) represented as a waveform, can be used to control the timing and level of ventilatory assist or simply monitor neural respiratory drive and breathing pattern (12). The Edi waveform is produced by capturing and processing EMG signals from the crural diaphragm in response to signals transmitted from the central respiratory center via the phrenic nerves, by placing a naso-gastric or oro-gastric Edi catheter embedded with sensors at the gastroesophageal junction. Specifically, the Edipeak value reflects the effort to generate tidal volume in response to neural stimulation via diaphragmatic contraction during the inspiratory phase and the Edimin value reflects the effort to maintain diaphragmatic contraction during the expiratory phase to preserve functional residual capacity (13). The NAVA is being increasingly recognized for its usefulness in providing synchronized and gentle [(such as reduction in peak inspiratory pressure and fraction of inspired oxygen [FiO2)] (14, 15) ventilatory support to neonates.
2. Objectives
In this study, we aimed to assess the changes in diaphragmatic activity in response to the administration of caffeine citrate in preterm infants using the Edi technology in NAVA. Specifically, the variables of interest were Edipeak and Edimin values before and after loading and maintenance dose administration of caffeine citrate.
3. Methods
3.1. Patient Enrollment
This prospective observational study included infants born at a gestational age of < 34 weeks, who were admitted to a level-IV NICU in South Korea between June 2022 and June 2023, received respiratory support by invasive or non-invasive NAVA, and were administered caffeine citrate, by convenience sampling. As per unit strategy, intravenous caffeine citrate was prophylactically administered in spontaneous-breathing preterm infants born at a gestational age of ≤ 29 weeks or was administered when the infant presented apnea not attributable to other confirmed/suspected etiology (such as sepsis, massive intraventricular hemorrhage, or mechanical obstruction of the airway). We commonly administer 20 mg/kg as the loading dose, and maintenance doses between 5 and 20 mg/kg/day are chosen as per the decision of the physician in charge. The ventilator settings were decided based on the clinical respiratory status observed by the physician (such as intercostal retraction, labored breathing, and percutaneous oxygen saturation). The study was conducted according to the 1975 Declaration of Helsinki. It was approved by the institutional review board of our hospital (approval number: KC22OISI0314). Informed parental consent was obtained before the initial dose of caffeine citrate was administered. The exclusion criteria included infants with major congenital anomalies, including structural or neuromuscular abnormalities, or those prenatally diagnosed or suspected with a genetic or chromosomal abnormality.
Servo-i® with NAVA® or Servo-n® (both Maquet Critical Care, Sweden) mechanical ventilators were used. Edipeak and Edimin values were retrieved from the NAVA software data at the following time intervals: Twenty minutes before starting caffeine citrate administration to 20 minutes after the completion of the loading dose, from 20 minutes before to 20 minutes after the administration of the first maintenance dose, and from 20 minutes before to 48 hours after the discontinuation of caffeine citrate. Considering the half time described in previous literature (16, 17), the discontinuation time point of caffeine citrate was defined as anywhere within 48 to 96 hours after the last dose was administered, without additional doses administered thereafter. Neocaf injection (20 mg/1 mL/vial or 60 mg/3 mL/vial, Pharmbio Korea Inc., South Korea) was used for loading and initial maintenance doses, and when the physician deemed that the infant could tolerate oral agents based on the enteral feeding achievement trajectory, this was changed to Neocaf oral solution (20 mg/1 mL/vial or 40 mg/2 mL/vial, Pharmbio Korea Inc., South Korea).
3.2. Data Collection
The baseline demographic data such as gestational age (weeks), birthweight (g), sex, maternal age (years), maternal diabetes, maternal hypertension, histologic chorioamnionitis, and preterm premature rupture of membrane (h) were obtained. Neonatal morbidity including respiratory distress syndrome (RDS) was identified. RDS was defined as respiratory distress symptoms and signs such as tachypnea, grunting, labored breathing, chest retraction, and need for an FiO2 of ≥ 0.4 to maintain a percutaneous oxygen saturation of ≥ 95%, accompanied by a ground-glass appearance, haziness of the lungs, air-bronchograms, or total white-out of lung fields on imaging.
The data on the following variables on caffeine citrate administration and respiratory support were also collected: Age (hours after birth) at administration of caffeine citrate loading dose and first maintenance dose, amount of caffeine citrate loading (mg/kg) and first maintenance doses (mg/kg), total duration of caffeine citrate administration (days), cumulative dose of caffeine citrate during hospital stay per day (mg/day), ventilator mode (invasive or non-invasive NAVA), FiO2, and NAVA level at the time of Edi-value retrieval. Data on heart rate (beats/min), number of apnea episodes, bradycardia (heart rate < 100/min), and desaturation (oxygen saturation < 85%) were collected before and after caffeine citrate discontinuation.
3.3. Statistical Analysis
Numerical values are presented as mean ± standard deviation or median (interquartile range) for continuous variables and number (percentage) for categorical variables. Wilcoxon signed-rank test was performed to analyze the changes in Edipeak, Edimin, neural respiratory rate (nRR), and back up (BU, %) changes before and after administration of caffeine citrate loading and maintenance doses. Spearman’s correlation analysis was performed to evaluate the correlation between Edipeak, Edimin, nRR, and BU. Mann–Whitney U test was used to compare Edi values depending on perinatal factors (such as gestational age, birthweight, and ventilator settings). SPSS version 29 (Armonk, NY, USA) was used for analysis; a P-value of < 0.05 was considered significant.
4. Results
A total of 14 infants were screened for enrollment, and after eliminating one due to informed consent withdrawal, 13 were included in the final analysis. Baseline characteristics of the included infants are presented in Table 1.
| Characteristic | Values |
|---|---|
| Gestational age (wk) | 28.8 ± 2.1 |
| Birthweight (g) | 1231 ± 441 |
| Male | 7 (53.8) |
| 1-min apgar | 4 [1 - 8] |
| 5-min apgar | 7 [2 - 9] |
| Cesarean delivery | 11 (84.6) |
| Maternal diabetes | 2 (15.4) |
| Maternal hypertension | 2 (15.4) |
| PPROM | 6 (46.2) |
| Oligohydramnios | 2 (15.4) |
| Histologic chorioamnionitis | 6 (46.2) |
| Antenatal corticosteroid | 12 (92.3) |
| Surfactant administration | 7 (53.8) |
| Age at caffeine loading-dose administration (h) | 3.258 ± 4.494 |
| Caffeine loading dose (mg/kg) | 19.924 ± 0.264 |
| Age at caffeine maintenance dose administration (h) | 26.965 ± 9.199 |
| Caffeine maintenance dose (mg/kg) | 8.840 ± 2.190 |
| PMA at caffeine cessation (wk) | 36.8 ± 2.6 |
| Ventilatory mode | 13 (100.0) |
| Invasive NAVA | 5 (38.5) |
| NIV-NAVA | 8 (61.5) |
Abbreviations: NAVA, neurally adjusted ventilatory assist; NIV, non-invasive; PMA, postmenstrual age; PPROM, preterm premature rupture of membranes.
a Values are expressed as No. (%), median [ranges], or mean ± SD.
Appendix 1 in Supplementary File describes the Edipeak, Edimin, nRR, and BU rate changes before and after administration of caffeine citrate loading and maintenance doses. Median Edipeak and Edimin values tended to be higher after the administration of the caffeine citrate loading dose than those before. nRR was similar at different measurement time points, and the BU rate showed insignificant and inconsistent changes after caffeine citrate loading- or maintenance-dose administration.
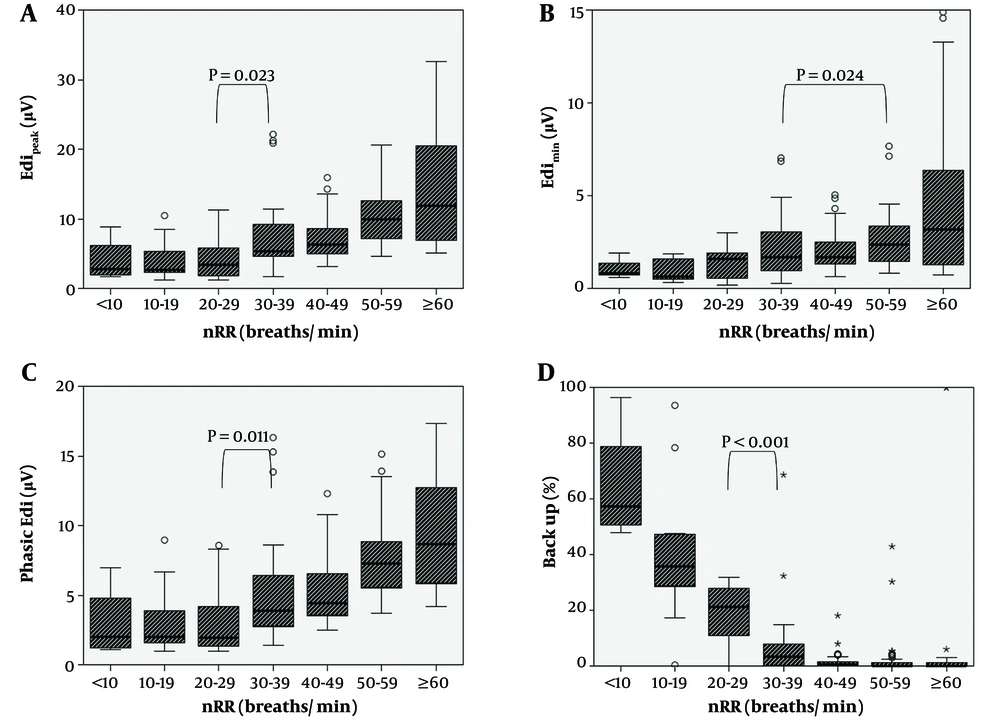
Spearman’s correlation analysis (Appendix 2 in Supplementary File) revealed a moderate positive correlation between nRR and Edipeak, Edimin, and phasic Edi (Edipeak-Edimin) values, while nRR and BU (%) showed a negative correlation (all P < 0.001). Figure 1 shows the Edi values and BU rate according to the nRR. Overall, Edipeak, Edimin, and phasic Edi tended to increase when the nRR (breaths/min) was ≥ 30 (Figure 1A - C). In contrast, the BU rate significantly declined as the nRR increased up to 30 - 39 breaths/min (P < 0.001), and the decline blunted thereafter (Figure 1D).
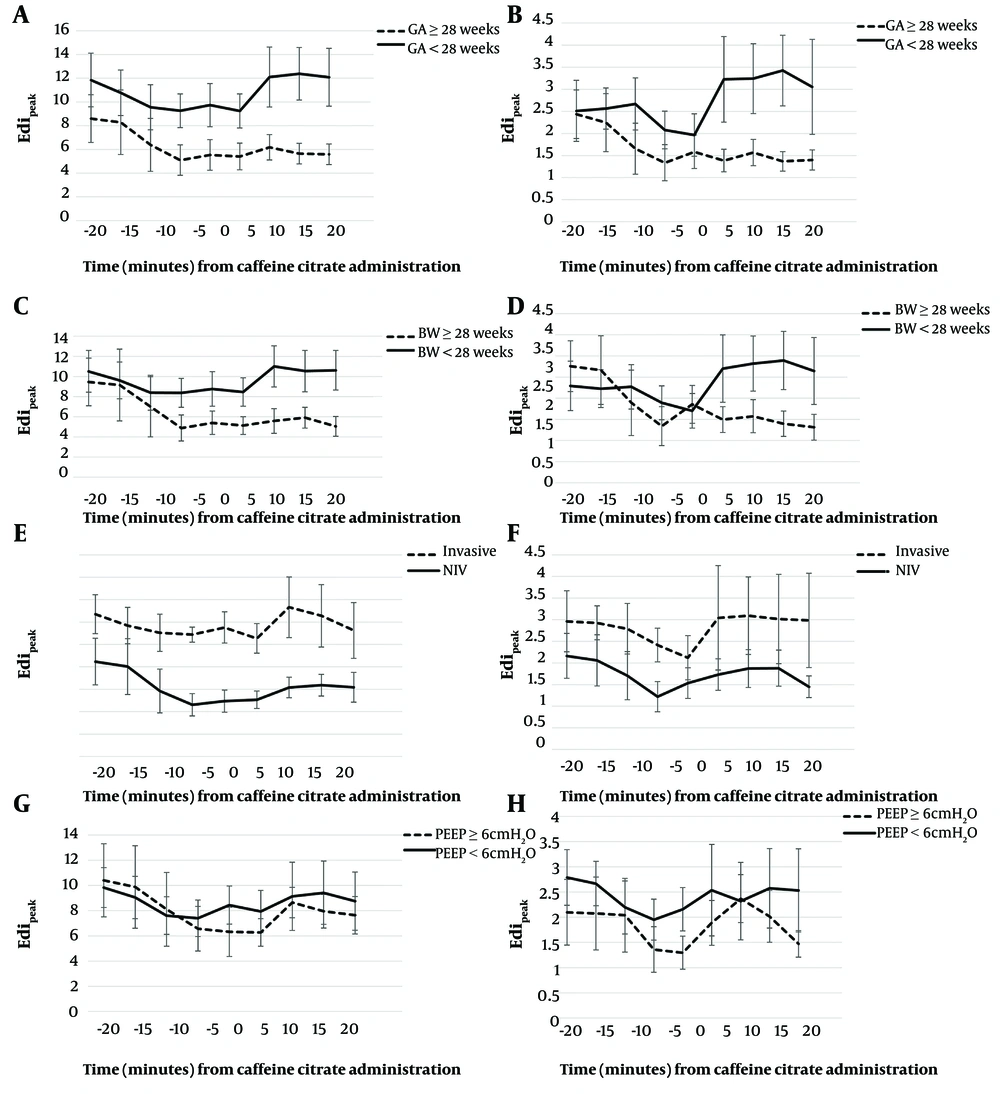
Appendix 3 in Supplementary File describes the Edipeak and Edimin values before and after caffeine citrate maintenance dose administration according to perinatal and postnatal factors. The post-maintenance Edipeak values were significantly higher in infants with a lower gestation, lower birthweight, and on invasive NAVA support compared to their counterparts. The post-maintenance Edimin values were significantly higher in infants receiving lower positive end-expiratory pressure (PEEP) of < 6 cmH2O than those receiving higher PEEP. Figure 2A - H presents the changing trend of Edipeak and Edimin values in 5-minute intervals before and after the administration of caffeine citrate maintenance doses according to different perinatal factors.
Comparison of Edipeak and Edimin according to perinatal factors. A and B, GA; C, and D, BW; E and F, ventilatory mode; G and H, PEEP level. Abbreviations: BW, birthweight; GA, gestational age; NIV, non-invasive; PEEP, positive end-expiratory pressure; Edi, electrical activity of the diaphragm.
Changes in Edipeak and Edimin values according to different durations and cumulative doses of caffeine citrate administered are shown in Appendix 4 in Supplementary File. Edipeak values tended to increase at 24 - 48 hours after caffeine citrate discontinuation compared with those measured at 24 hours of discontinuation, regardless of the duration or cumulative dose. When caffeine citrate was stopped at < 36-week PMA, Edipeak values showed an increasing trend at 24 - 48 hours rather than during the first 24 hours of discontinuation; however, they tended to decrease in those with caffeine citrate discontinuation at ≥ 36-week PMA.
Caffeine citrate discontinuation resulted in no significant changes in heart rate; however, the number of apnea and desaturation episodes significantly increased during the 48 hours after discontinuation (Appendix 5 in Supplementary File).
5. Discussion
Edipeak and Edimin values tended to increase after caffeine citrate loading-dose administration. Edi values were dependent on whether neural breathing was intact, presenting an increasing trend when the nRR was ≥ 30, compared with lower rates. The Edi values showed characteristic trends depending on several perinatal and postnatal factors such as gestational age, postnatal lung condition, or ventilatory modes/settings applied. Moreover, PMA at caffeine citrate discontinuation seemed to affect Edipeak in different directions.
The mechanism of action of caffeine citrate includes stimulation of the respiratory center in the medulla, increasing sensitivity to carbon dioxide, increasing skeletal muscle tone, and enhancing diaphragmatic contractibility (7). Few studies (8, 9) have reported on the use of dEMG to measure the electrical activity of the diaphragm after caffeine citrate administration in neonates. Williams et al. (8) observed that the dEMG amplitude peaked at 20 minutes after caffeine citrate loading-dose administration in preparation for extubation, in preterm infants born at < 34-week gestation. However, according to van Leuteren et al. (10), the dEMG values in preterm infants can yield variable results depending on the location of device attachment and the respiratory rate of the infant, thus limiting the accuracy of dEMG-derived measurements.
In comparison, NAVA, which uses an Edi catheter that senses the electrical stimulation arousing diaphragmatic contraction, enables direct observation of the electrical signal from the respiratory center (18) in both invasive (intubated) and non-invasive (non-intubated) modalities (19-21). Guidance in assessing the location where the electrical signal has been sensed is available, enabling frequent confirmation of the adequacy of catheter-insertion depth, which enhances the reliability of the Edi measurements obtained. Only one study to date has reported on the effect of caffeine citrate loading-dose administration in central apnea using the Edi technology in NAVA-mounted ventilators; however, the loading dose was administered at a later age (mean age of 3 postnatal days) and the effect of maintenance dose(s) administration or agent cessation was not analyzed compared with our study (22).
In the present study, Edipeak and Edimin values were within the previously described ranges (12, 23). Overall, both increased after administration of the caffeine citrate loading dose. When the nRR is ≥ 30/min, increases in Edipeak, Edimin, and phasic Edi, with a decrease in BU%, can be interpreted as a result of increased respiratory drive and effort to breathe due to the effects of caffeine. Despite statistical insignificance in a predominant portion of our study results due to the small sample size, they are consistent with previous studies that demonstrated increasing diaphragmatic activity via ultrasound (24) or Edi technology after caffeine citrate administration (8, 22). This trend may be attributed to the respiratory center-stimulatory action of caffeine citrate, resulting in increased neural respiratory drive and diaphragmatic activation. This phenomenon seems to be better observed in infants born at < 28-week gestation or those with birthweights < 1250 g, possibly due to the underlying respiratory prematurity with insufficient respiratory drive which is stimulated by the action of caffeine citrate.
The Edi values tended to increase after caffeine citrate administration in infants on invasive NAVA support, which could be attributed to the fact that those who have more unfavorable postnatal lung conditions necessitating intubation would likely have been more premature and of lesser weight.
The Edi levels tended to increase after caffeine citrate loading-dose administration when the PEEP was set at ≥ 6 cmH2O or the NAVA level was set at ≥ 1.5. This may imply that appropriate ventilator settings may not only provide the set respiratory support, but also possibly maximize the action of the medication used. Although optimal PEEP levels in preterm infants have not been established due to insufficient evidence (25), some expert opinions and research mention the importance of a PEEP sufficiently high to recruit alveoli (26, 27) and maintain functional residual capacity. Provision of appropriate PEEP levels may not only be important for preventing alveolar collapse to reduce diaphragmatic tension, but also for maximizing the inspiratory contraction activity via the effect of caffeine citrate.
Regarding the Edi values after caffeine citrate discontinuation, the Edipeak showed an increasing trend regardless of the duration or cumulative dose of caffeine citrate administered. On discontinuation of caffeine citrate, which stimulates the central respiratory system, its effects gradually diminish over time, leading to an increase in apnea and hypoxemic episodes. Since the half-life of caffeine citrate in preterm infants can extend from 48 to 96 hours (16, 17), its effects would have waned by 24 - 48 hours after discontinuation compared to the first 24 hours. Consequently, a rebound in apnea/desaturation episodes may occur more frequently during this later period. The increased respiratory effort in response may have been reflected in the rise of Edipeak values in the later hours of drug discontinuation. Meanwhile, a similar result was observed only in infants who stopped the medication before a PMA of 36 weeks but not in those who stopped at a PMA of ≥ 36 weeks. This trend may be attributed to the different maturational status of respiratory muscles depending on the PMA of preterm infants. While it may be an indicator of active diaphragmatic contraction, it may also be attributed to a greater breathing workload. Our findings are insufficient to determine the age at which discontinuation of caffeine administration is safe, and considering the wide variation in policy for the time point of caffeine citrate discontinuation in different institutes (28), monitoring the number of episodes of bradycardia and/or apnea for an increasing trend after caffeine citrate discontinuation is important, particularly if it occurs prior to term-equivalent age or depending on the level of respiratory support provided.
This study has some limitations. First, the small sample size resulted in a restricted analysis largely based on trends and not strict statistical significance. Because the patients did not undergo randomization for the study, we tried to reduce selection bias by using the consecutive sampling method. However, only two ventilators were equipped with NAVA software programs, and the infants stayed on NAVA support even after the initial measurements for the study were completed, throughout the time the physician deemed it necessary, since it would be unethical to withhold required ventilatory support for the infants. Hence, only limited number of patients could be enrolled due to the lack of ventilator availability. Second, although we sought to assess the effect of caffeine citrate on Edi changes, the infants enrolled in our study were administrated the medication relatively early during the postnatal period compared with previous studies (8, 22). Since various dynamic changes due to active procedures and treatment (such as surfactant replacement) occurred during these hours, in addition to normal neural respiratory transition (23), they may have conferred confounding effects, making it more challenging to yield significant results. Third, whether the caffeine citrate levels were within the therapeutic range when the Edi values were obtained was not assessed in this study. As most enrolled patients were very preterm infants, repetitive blood-sample collection from this vulnerable population was considered invasive. Moreover, other means of measurement (such as saliva) (29) were technologically limited. Finally, we defined the point of caffeine discontinuation as a range and not one time point. This was based on the observation that the half-life of caffeine citrate is variable (16, 17). Furthermore, when considering the working environment of the NICU, the Edi catheter re-insertion was deemed safer under the supervision of a study team member for the purpose of measurement and not for urgent respiratory support as in the immediate postnatal period. However, long-term measurements beyond the predetermined range were not included in the study protocol to maximize the ventilator availability for future patients, which resulted in our inability to assess the sustained effects of drug cessation.
Despite the lack of definitive statistical significance due to the limited sample size, this study provides valuable insights on the effects of caffeine citrate on diaphragmatic activity in preterm infants, as reflected in increased Edipeak and Edimin values following caffeine administration. These changes, particularly prominent in infants with greater respiratory immaturity and on invasive NAVA support, highlight the role of caffeine in enhancing respiratory drive. The influence of ventilatory settings such as PEEP on Edi values suggests that appropriate respiratory support may augment the effects of caffeine. The rebound in Edipeak values after caffeine cessation, especially in infants with lower PMA, underscores the need for close monitoring post-discontinuation, which has not been concretely discussed in previous studies. Although limited by statistical power, these findings provide preliminary data on the impact of caffeine on neonatal respiratory function, based on Edi values and possibly offer a foundation for future research to optimize treatment strategies. The small sample size was determined by practical constraints rather than by a priori power analysis. Consequently, the power of the study may not have been sufficient to detect statistically significant differences. Nonetheless, the trends observed in the Edi values in relation to caffeine administration/cessation suggest clinically relevant changes that warrant further investigation in larger, adequately powered studies. Additionally, a tailored design may provide more conclusive results and suggest an optimal administration timing and dose range, which remain unclear (30, 31), to maximize therapeutic effects while minimizing side effects. The scope of future studies could be extended to delineating diaphragmatic activity changes impacted by not only caffeine citrate, but other agents (such as endotracheal surfactant therapy) or treatment modalities (such as different ventilator modes/settings or weaning protocols) for improving respiration.
5.1. Conclusions
Edipeak and Edimin, which show variable values depending on intact neural breathing, tended to increase following caffeine citrate loading-dose administration. The Edi values showed different trends depending on perinatal factors and ventilatory support settings. Moreover, our study indicates that it may be particularly prudent to monitor changes in clinical symptoms in infants with near-term PMA after caffeine citrate cessation.