1. Background
Hydrocephalus is a common condition encountered in neurosurgery, characterized by the abnormal accumulation of cerebrospinal fluid (CSF) within the brain's ventricular system, with or without abnormal dilation of the ventricular system (1). Its presentation ranges from primary congenital hydrocephalus of indeterminate etiology to secondary acquired hydrocephalus resulting from conditions such as intracranial hemorrhage, traumatic brain injury, brain tumors, or intracranial infections (2). In addition to classifying hydrocephalus based on etiology, clinical classification can also be performed based on the underlying cause of CSF accumulation. Broadly, hydrocephalus is classified as obstructive when obstruction occurs above the fourth ventricular outlet plane, or communicating when obstruction is absent. This classification is significant as it determines the preferred surgical approach. Furthermore, hydrocephalus can be classified based on patient age into pediatric and adult hydrocephalus, based on the development speed into acute and chronic hydrocephalus, and based on intracranial pressure into normal-pressure hydrocephalus and high-pressure hydrocephalus (3). In summary, the various classification methods serve the purpose of employing different strategies to better treat patients, alleviate symptoms, improve quality of life, and reduce mortality and morbidity. Each classification method has its unique significance, guiding the treatment of hydrocephalus from different perspectives. However, we propose a new classification of hydrocephalus into simple hydrocephalus and complex hydrocephalus, aiming to guide neurosurgeons in better treating patients in the face of unique clinical situations. Currently, the treatment strategy for complex hydrocephalus is imperfect. Often, during blind treatment, patients experience repeated complications and undergo multiple operations. Although they may eventually be cured, the long-term prognosis is poor. Therefore, it is necessary to develop a new surgical management procedure to reduce the number of operations for complex hydrocephalus and improve long-term outcome prognosis.
We believe that complex hydrocephalus is defined not by age and development speed, nor by the condition of obstruction and intracranial pressure, but rather by surgical outcomes, etiology, and anatomical structure. Here, we propose a definition of complex hydrocephalus. Firstly, hydrocephalus requiring multiple surgeries to cure must be considered complex hydrocephalus. These multiple surgeries refer to the repeated use of the same procedure, such as ventriculoperitoneal shunt (VPS) (4), or the sequential use of different procedures, such as VPS, ventriculoatrial shunt (VAS) (5), endoscopic third ventriculostomy (ETV) (6), and choroid plexus cauterization (CPC) (7). Secondly, hydrocephalus caused by hemorrhage (8) and/or infection (9) is also considered complex hydrocephalus. This is because the presence of blood or pathogenic microorganisms accumulating in the ventricles not only affects the normal ventricular structure but also affects the patient's overall condition, leading to the failure of the initial surgery. Thirdly, multiloculated hydrocephalus is also considered complex hydrocephalus (10). The formation of fibrous septa within the ventricles divides the originally intact and unobstructed ventricles into separate chambers, making it impossible to resolve hydrocephalus with a single shunt surgery, necessitating multiple surgeries to open up the ventricles and subsequently divert cerebrospinal fluid. Fourthly, preterm infants with very low birth weight are prone to germinal matrix hemorrhage due to preterm birth and extremely low weight. Because the gestational age is too small and the weight is too light, the tolerance to surgery is poor, making it more difficult to manage (11). Finally, there are also difficult-to-treat conditions considered complex hydrocephalus, such as stenosis of the aqueduct of Sylvius or Dandy-Walker malformation. In addition to these types mentioned above, the rest of the hydrocephalus is considered simple hydrocephalus. This is because this type of hydrocephalus is often easy to identify, can be well controlled with a single operation, and has a good prognosis. According to the above definition, complex hydrocephalus can arise from two situations: Firstly, simple hydrocephalus that has not been properly managed develops into complex hydrocephalus due to various uncontrollable reasons; secondly, complex hydrocephalus can be diagnosed initially, which is preventable and controllable if detected early. If doctors detect and diagnose early and adopt personalized treatment approaches tailored to the individual patient's condition, it is still possible to successfully cure patients while minimizing the number of surgeries. At present, researchers' understanding of complex hydrocephalus is not comprehensive enough. Currently, no scholars have proposed the most accurate definition, nor have they presented a systematic procedure for surgical management (12). Without considering the underlying causes of complex hydrocephalus or even being able to predict whether a patient has complex hydrocephalus, there is a tendency to blindly adopt current mainstream surgical techniques or merely pursue the glorification of endoscopic surgery. Therefore, the current treatment outcomes are not satisfactory. Without careful analysis of the patient's etiology, blindly carrying out surgery will not only fail to solve the patient's problem but will further complicate the condition. Many surgical failures are often accompanied by various complications, including but not limited to bleeding, infection, shunt blockage, and shunt rupture.
Although epidemiological data on complex hydrocephalus are still incomplete (13), we can draw conclusions from its etiology. Its incidence rate is relatively high, posing a common challenge for neurosurgeons worldwide. Moreover, without proper treatment, the mortality and disability rates among patients are considerable, leading to a significant decline in their quality of life. Therefore, our team extensively reviewed the literature and combined it with our clinical experience to provide a more comprehensive summary of complex hydrocephalus. We aim to systematically propose relevant concepts and provide a relatively appropriate treatment protocol, emphasizing early identification of complex hydrocephalus and the implementation of personalized treatment measures. Based on this, a two-stage surgery is adopted, with complexity factors controlled in the first stage, allowing patients to undergo subsequent surgery smoothly. In the second stage, the most suitable surgical form is selected to completely treat the hydrocephalus based on its presentation.
2. Objectives
In this study, we retrospectively reviewed patients with hydrocephalus in our hospital over the past five years. We screened patients with complex hydrocephalus according to the definition proposed earlier in the text, focusing particularly on children. The rationale behind this decision is twofold: First, children have a higher incidence rate compared to adults, especially preterm infants, who not only exhibit hydrocephalus but also meet the criteria for complex hydrocephalus. Besides this medical aspect, socially, children carry the hopes of their families. When dealing with pediatric patients, neurosurgeons often bear a heavier psychological burden, as there is a more urgent desire to successfully treat them. Therefore, this paper analyzed the medical records of pediatric patients with complex hydrocephalus in our hospital over the past five years. We showcased typical cases while conducting a literature review. Drawing upon our clinical experience, we proposed a systematic surgical diagnostic and treatment protocol for early identification and intervention to improve the overall treatment level of children with complex hydrocephalus and enhance the prognosis and quality of life of patients.
3. Methods
3.1. Patient Selection
We retrieved data of patients with hydrocephalus in final diagnoses from our hospital, which is the largest treatment center for hydrocephalus in the central south region of China. First, we filtered patients admitted between January 2019 and January 2024 based on their admission dates. Then, we further screened based on age at admission, selecting patients younger than 14 years old. Finally, according to the medical records and the definition of complex hydrocephalus mentioned earlier, we identified 114 cases of pediatric complex hydrocephalus. These patients were then divided into two groups based on whether they received the new surgical management procedure proposed by us: The new group and the old group, with the old group serving as a control. Since this study involved retrospective analysis using anonymized data, it poses no risk of breaching patient confidentiality or causing iatrogenic harm; thus, there is no need for approval from the ethics committee or informed consent from patients.
3.2. Data Collection
Comprehensive patient data, including medical histories, surgical procedures, radiological findings, outcomes, and follow-up information, were retrieved from the medical record management system at Xiangya Hospital, Central South University. Key data include gender, age, whether the patient is a preterm infant with very low birth weight, presence of intracranial hemorrhage or infection, presence of multiloculated hydrocephalus, and details of surgeries performed, including the number and type of surgeries. Additionally, follow-up time and ultimate therapeutic outcomes, encompassing both symptomatic improvement and radiological resolution of ventricular system abnormalities, were recorded. Moreover, long-term prognostic indicators, including cognitive, developmental, and functional outcomes, as well as complication rates, reoperation rates, and survival rates, were documented.
3.3. Statistical Analysis
Means and standard deviations were used to represent continuous variables, while frequencies and percentages were used to represent categorical variables. A t-test was used for measurement data, and a chi-square test was used for categorical data. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Demographics and Etiologies of 114 Patients in Two Groups
After careful screening, a total of 114 pediatric patients with complex hydrocephalus were included in this study. Of these, 40 patients received the new surgical management procedure, while 74 followed the original process as a control group. In the new group, there were 21 males and 19 females. The age distribution included 3 patients younger than 1 month, 20 patients aged between 1 month and 1 year, and 17 patients aged between 1 and 14 years. Among them, 8 had a history of being preterm infants with very low birth weight, 18 presented with intracranial hemorrhage, 20 developed intracranial infection, 10 exhibited multiloculated structures, and 8 had undergone previous surgeries. In the old group, serving as a control, there were 51 males and 23 females. The age distribution included 2 patients younger than 1 month, 18 patients aged between 1 month and 1 year, and 54 patients aged between 1 and 14 years. Among them, 12 had a history of being preterm infants with very low birth weight, 36 presented with intracranial hemorrhage, 39 developed intracranial infection, 14 exhibited multiloculated structures, and 18 had undergone previous surgeries (Table 1).
| Variables | New | Old | Sum |
|---|---|---|---|
| Gender; No. (%) | |||
| Male | 21 (52.5) | 51 (68.9) | 72 (63.2) |
| Female | 19 (47.5) | 23 (31.1) | 42 (36.8) |
| Age; No. (%) [mean ± SD] (range) | |||
| 0 - 1 (mo) | 3 (7.5) [13.0 ± 8.9] (6 - 23) | 2 (2.7) [13.5 ± 19.1] (0 - 27) | 5 (4.4) [13.2 ± 11.4] (0 - 27) |
| 1 - 12 (mo) | 20 (50.0) [5.6 ± 2.6] (2 - 11) | 18 (24.3) [5.7 ± 3.5] (2 - 11) | 38 (33.3) [6 ± 2.8] (2 - 11) |
| 1 - 14 (y) | 17 (42.5) [4.6 ± 3.7] (1 - 12) | 54 (73.0) [5.2 ± 3.9] (1 - 13) | 71 (62.3) [5.0 ± 3.8] (1 - 13) |
| Premature with VLBW; No. (%) | 8 (20.0) | 12 (16.2) | 20 (17.5) |
| Hemorrhage | 18 (45.0) | 36 (48.6) | 54 (47.4) |
| Infection | 20 (50.0) | 39 (52.7) | 59 (51.8) |
| Multiloculated | 10 (25.0) | 14 (18.9) | 24 (21.1) |
| Surgery performed | 8 (20.0) | 18 (24.3) | 26 (22.8) |
Abbreviation: VLBW, very low birth weight.
a New refers group of patients treated with new procedure of surgical management, old refers group of patients treated with old procedure of surgical management.
4.2. Surgical Management of 114 patients in Two Groups
The average number of surgeries performed in the new group was 1.4 ± 0.7, with a maximum of 4 surgeries. In this group, 10 patients underwent extra ventricular drainage (EVD) with an Ommaya reservoir, 4 underwent cyst fenestration, 37 underwent VPS, 0 underwent VAS, 2 underwent ETV, and 0 underwent CPC. Additionally, 2 patients underwent other surgeries to address the etiologies. In the old group, serving as a control, the average number of surgeries performed was 3.0 ± 1.2, with a maximum of 7 surgeries. In this group, 25 patients underwent EVD with an Ommaya reservoir, 18 underwent cyst fenestration, 54 underwent VPS, 5 underwent VAS, 23 underwent ETV, and 17 underwent CPC. Additionally, 82 patients underwent other surgeries to address the etiologies. A t-test was conducted to compare the difference in the average number of surgeries between the new group and the old group. The t-value was 7.985, and the P-value was less than 0.01, indicating a significant difference between the two groups. This result suggests that the new surgical management procedure significantly reduced the number of operations in children with complex hydrocephalus (Table 2).
| Surgical Type | New | Old | Sum |
|---|---|---|---|
| EVD with Ommaya capsule | 10 (18.2) | 25 (11.2) | 35 (12.5) |
| Cyst fenestration | 4 (7.3) | 18 (8.0) | 22 (7.9) |
| VPS | 37 (67.3) | 54 (24.1) | 91 (32.6) |
| VAS | 0 (0) | 5 (2.2) | 5 (1.8) |
| ETV | 2 (3.6) | 23 (10.3) | 25 (9.0) |
| CPC | 0 (0) | 17 (7.6) | 17 (6.1) |
| Other | 2 (3.6) | 82 (36.6) | 84 (30.1) |
| Number of surgeries; mean ± SD (range)c | 1.4 ± 0.7 (1 - 4) | 3.0 ± 1.2 ( 2 - 7) | 2.4 ± 1.4 (1 - 7) |
Abbreviations: EVD, extra ventricular drainage; VPS, ventriculoperitoneal shunt; VAS , ventriculoatrial shunt; ETV, endoscopic third ventriculostomy; CPC, choroid plexus cauterization.
a Values are expressed as No. (%) unless otherwise indicated.
b New refers group of patients treated with new procedure of surgical management, old refers group of patients treated with old procedure of surgical management.
ct = 7.985, P < 0.01.
4.3. Follow-up and Long-term Outcomes of 114 Patients in Two Groups
After a mean follow-up time of 5.4 months in the new group and 5.5 months in the old group, ultimately, in the new group, 40 patients showed symptom relief and improvement, while 30 patients demonstrated normalization of the ventricular system on imaging. In the old group, 73 patients showed symptom relief and improvement, while 49 patients demonstrated normalization of the ventricular system on imaging. After comparison by chi-square test, the P-values for these two data points between the two groups were 0.460 and 0.332, indicating no significant differences. This suggests that regardless of the surgical management procedure adopted, patients will eventually be cured, which is the purpose of surgical treatment. Therefore, there is no difference in these two indicators.
However, considering the patient as a child, long-term prognosis is of extremely important value. A relatively simple and direct method is chosen to evaluate whether it is normal or not, based on the experience of clinicians, instead of using more complicated scales to quantify it. In the new group, 37 patients were cognitively normal, 36 were developmentally normal, 35 were functionally normal, 7 had complications, 7 underwent reoperations, and 40 survived. In the old group, 57 patients were cognitively normal, 53 were developmentally normal, 52 were functionally normal, 74 had complications, 74 underwent reoperations, and 73 survived. After the chi-square test, except for the survival rate, which is not different from the cure effect mentioned earlier, the other long-term prognostic outcome indicators, complication rate, and reoperation rate showed significant changes, with P-values all less than 0.05, and some results less than 0.01. This indicates that the new surgical management procedure contributes to a better long-term prognosis by reducing the number of operations (Table 3).
| Variables | New | Old | χ2 | P-Value |
|---|---|---|---|---|
| Follow-up duration (mo) | 5.4 ± 1.7 | 5.5 ± 1.9 | - | - |
| Relief of symptom | 40 (100) | 73 (98.6) | 0.545 | 0.460 |
| Normalization of ventricular system on imaging | 30 (75.0) | 49 (66.2) | 0.942 | 0.332 |
| Cognition normal | 37 (92.5) | 57 (77.0) | 4.297 | 0.038 |
| Developmental normal | 36 (90.0) | 53 (71.6) | 5.122 | 0.024 |
| Functional normal | 35 (87.5) | 52 (70.3) | 4.265 | 0.039 |
| Complication | 7 (17.5) | 74 (100) | 85.92 | < 0.01 |
| Reoperation | 7 (17.5) | 74 (100) | 85.92 | < 0.01 |
| Survival | 40 (100) | 73 (98.6) | 0.545 | 0.460 |
a Values are expressed as No. (%) or mean ± SD.
b New refers group of patients treated with new procedure of surgical management, old refers group of patients treated with old procedure of surgical management.
4.4. Different Responsiveness to Surgery Between Premature with Very Low Birth Weight and Full-term Infant
We mentioned that being a premature infant is an important factor in complex hydrocephalus, which holds clinical value for comparison with full-term infants. Here, we compared the difference in response to surgery between 20 premature infants and 94 full-term infants. In premature infants, the average number of surgeries performed is 2.5 ± 1.1, while it is 2.4 ± 1.4 in full-term infants, showing no significant difference. However, in terms of long-term outcomes, 19 premature infants showed symptom relief and improvement, 16 demonstrated normalization of the ventricular system on imaging, 13 were cognitively normal, 13 were developmentally normal, 11 were functionally normal, 16 had complications, 16 underwent reoperations, and 19 survived. In contrast, among full-term infants, 94 showed symptom relief and improvement, 63 demonstrated normalization of the ventricular system on imaging, 81 were cognitively normal, 76 were developmentally normal, 76 were functionally normal, 65 had complications, 65 underwent reoperations, and 94 survived. After the chi-square test, there were significant differences in symptom relief, cognitive normalcy, functional normalcy, and survival. Although other indexes did not show significant differences, there were still some differences. This reflects the difference in reactivity of premature infants to surgery (Table 4).
| Variables | Premature with VLBW | Full-term Infant | χ2 | P-Value |
|---|---|---|---|---|
| Number of surgeries | 2.5 ± 1.1 | 2.4 ± 1.4 | 0.010 c | 0.992 |
| Relief of symptom | 19 (95.0) | 94 (100) | 4.742 | 0.029 |
| Normalization of ventricular system on imaging | 16 (80.0) | 63 (67.0) | 1.306 | 0.253 |
| Cognition normal | 13 (65.0) | 81 (86.2) | 5.109 | 0.024 |
| Developmental normal | 13 (65.0) | 76 (80.9) | 2.420 | 0.120 |
| Functional normal | 11 (55.0) | 76 (80.9) | 6.097 | 0.014 |
| Complication | 16 (80.0) | 65 (69.1) | 0.944 | 0.331 |
| Reoperation | 16 (80.0) | 65 (69.1) | 0.944 | 0.331 |
| Survival | 19 (95.0) | 94 (100) | 4.742 | 0.029 |
Abbreviation: VLBW, very low birth weight.
a Values are expressed as No. (%) or mean ± SD.
b New refers group of patients treated with new procedure of surgical management, Old refers group of patients treated with old procedure of surgical management
ct-value for number of surgeries.
4.5. Procedure of Surgical Management
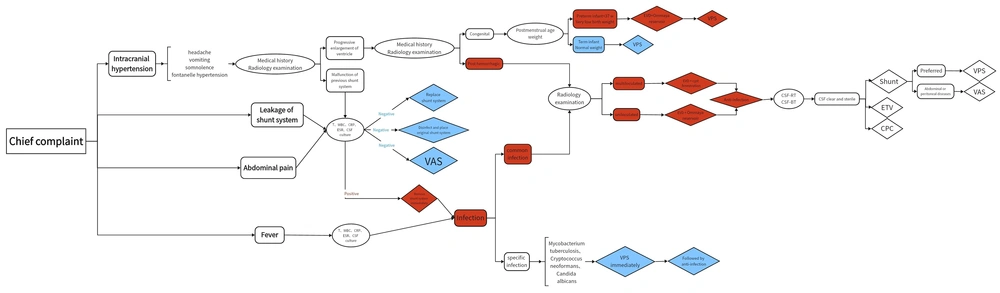
After retrieving and analyzing the treatment courses of pediatric patients with complex hydrocephalus in our institution and reviewing the literature, we attempted to summarize the management approach for complex hydrocephalus. Starting from the initial consultation, we assessed the patient's clinical presentation and analyzed potential causes, ultimately confirming complex hydrocephalus through appropriate investigations, as outlined in Figure 1. The purpose of such analysis and assessment is to evaluate the likelihood of progression to complex hydrocephalus in the early stages of patient presentation. For patients with high-risk factors, specific management strategies should be employed rather than resorting to surgery blindly. We selected the following three typical cases to support the validity of the surgical management procedure proposed by us.
A schematic diagram illustrating the procedure of surgical management proposed in this work. The content of the rounded rectangle represents different conditions. The contents of the ellipse indicate different methods to judge. The contents of the diamonds indicate different surgical procedures. Blue represents simple hydrocephalus while red represents complex hydrocephalus. Abbreviations: T, temperature; WBC, white blood cell; CRP, C-reaction protein; ESR, erythrocyte sedimentation rate; CSF, cerebrospinal fluid; CSF-RT, cerebrospinal fluid routine examination; CSF-BT, cerebrospinal fluid biochemical test; EVD, Extra ventricular drainage; VPS, ventriculoperitoneal shunt; VAS, ventriculoatrial shunt; ETV, endoscopic third ventriculostomy; CPC, choroid plexus cauterization.
4.5.1. Typical Case 1
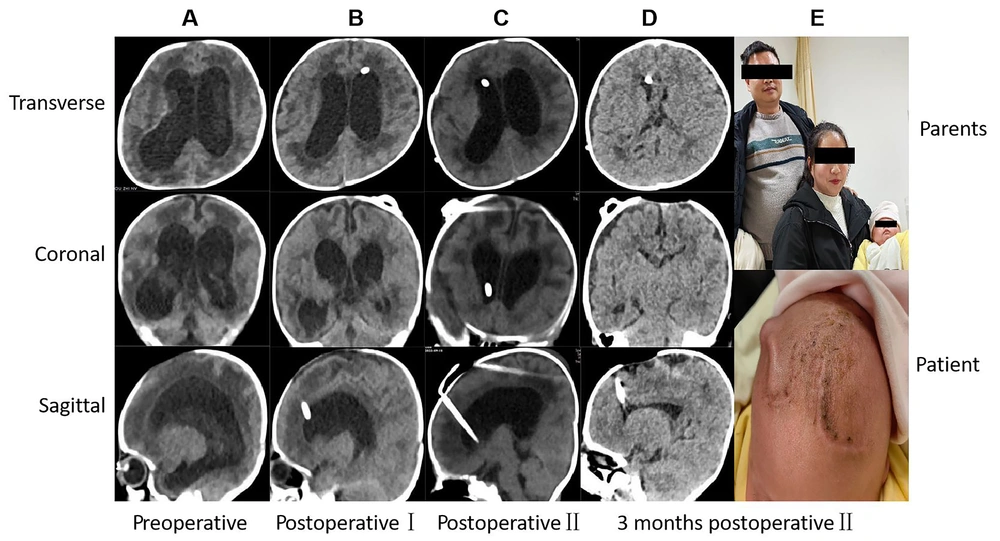
A 3-month-old male infant, born at full term with normal birth weight, developed intracranial hemorrhage complicated by intracranial infection shortly after birth. He underwent two surgeries at another hospital, which included EVD and Ommaya reservoir placement. Subsequently, cerebrospinal fluid culture confirmed infection with Acinetobacter baumannii. He was then referred to our hospital, where CT and MRI scans revealed enlargement of the ventricular system. The patient met two criteria for defining complex hydrocephalus: Unsuccessful single surgery and hemorrhage and/or infection. Therefore, a staged approach was adopted. In the first stage, EVD along with Ommaya reservoir placement was performed. This involved both medication injection into the capsule to cleanse the ventricles and direct external drainage of cerebrospinal fluid to control the existing hemorrhage and infection. Once the cerebrospinal fluid became clear, confirming that the hemorrhage had been absorbed and the infection controlled, the second stage involved VPS placement. Following surgery, the symptoms improved, and the patient was discharged. A follow-up CT-scan performed five months later revealed normalization of the ventricular system. The imaging results at each time point during surgical management are shown in Figure 2.
Radiology imaging at different time points during procedure of surgical management and postoperative follow-up for typical case 1, which had used new concepts of complex hydrocephalus. A, Three section of patient before operation Ⅰ showing the enlargement of ventricle; B, three section of patient after operation Ⅰ; C, three section of patient after operation Ⅱ; D and E, 5 months after operation Ⅱ showing the normal ventricles and the happiness of parents. Information of patient were erased to avoid leaking information.
4.5.2. Typical Case 2
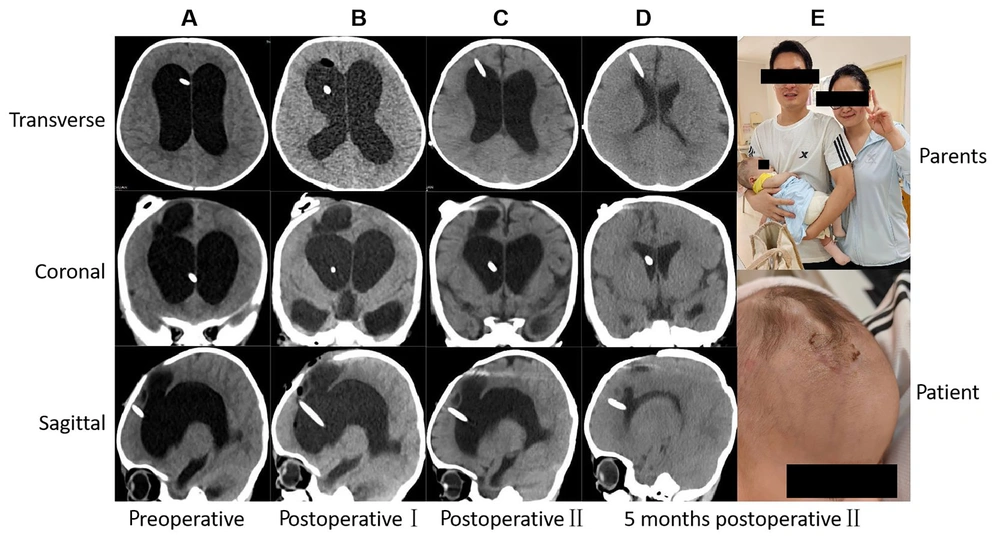
A 6-day-old female, born as a preterm infant with very low birth weight, with a corrected gestational age of 30 weeks and weighing 1.1 kilograms, developed intracranial hemorrhage shortly after birth, without evidence of intracranial infection. No surgery was performed initially, and she was diagnosed with intracranial hemorrhage before being referred to our hospital. Upon admission, a CT-scan revealed enlargement of the ventricular system. The patient met two criteria for defining complex hydrocephalus: Intracranial hemorrhage and prematurity with very low birth weight. Consequently, a staged approach was adopted. In the first stage, EVD along with Ommaya reservoir placement was performed. This involved both medication injection into the capsule to cleanse the ventricles and direct external drainage of cerebrospinal fluid to control the existing hemorrhage. Additionally, external drainage via the Ommaya reservoir was utilized to stabilize the patient's condition and allow time for the second stage surgery, ensuring that the patient reached a corrected gestational age of over 37 weeks and a weight exceeding 2.5 kilograms. Once the patient met the surgical criteria, the second stage involved VPS placement. Following surgery, symptoms improved, and the patient was discharged. A follow-up CT scan performed three months later showed normalization of the ventricular system. The imaging results at each time point during surgical management are shown in Figure 3.
Radiology imaging at different time points during procedure of surgical management and postoperative follow-up for typical case 2, which had used new concepts of complex hydrocephalus. A, Three section of patient before operation Ⅰ showing the enlargement of ventricle; B, three section of patient after operation Ⅰ. C, three section of patient after operation Ⅱ; D and E, 3 months after operation Ⅱ showing the normal ventricles and the happiness of parents. Information of patient were erased to avoid leaking information.
5. Discussion
Based on current advances in basic medical research and the development of translational medicine (14), the journey towards pharmacological intervention for hydrocephalus is arduous (15). This is due to the fact that hydrocephalus is not merely a single, well-defined etiological disease but rather a syndrome encompassing multiple etiologies with common clinical manifestations (16). Identifying drug targets relies heavily on continuous in-depth research by scientists (17). Therefore, the most effective treatment modality currently is surgical intervention (18). Surgical interventions can be broadly categorized into four main types, each serving different purposes: Relieving intraventricular obstruction, reducing cerebrospinal fluid secretion, establishing new intracranial circulation, and establishing extracranial shunting. Each of these categories has its own detailed subdivisions and specific operative techniques. The selection of a particular procedure depends on the patient's physical condition and the surgeon's proficiency in the technique. However, the contraindications for these surgeries often overlap and include conditions such as unresolved intracranial hemorrhage, uncontrolled intracranial infection, preterm neonates, and other systemic illnesses. These contraindications fall within the scope of the complex definition of hydrocephalus presented in this paper. Therefore, comprehensive preoperative assessment of the patient's condition, early identification, and prediction of the possible occurrence of complex hydrocephalus, and taking corresponding measures before surgery are crucial. This preoperative preparation should not be perceived as a single surgical failure but rather as a preparatory step for formal surgical treatment, serving as the cornerstone for a successful single surgical intervention. It avoids the prolonged suffering and adverse effects on the patient's physical and mental health, as well as their economic well-being, associated with repeated shunting procedures, fenestration, and cauterization.
The Ommaya reservoir, invented and first applied by Ayub Khan Ommaya in 1963 (19), has gradually gained widespread use in surgical procedures (20). This device offers unique advantages due to its small size, minimizing the impact of foreign bodies implanted in the patient's body and thus reducing iatrogenic damage. Importantly, compared to direct implantation of shunting tubes, the Ommaya reservoir minimizes the risks of blockage and infection, which are common causes of postoperative complications leading to complex hydrocephalus and significantly impacting patients. Moreover, the Ommaya reservoir allows for repeated drainage of cerebrospinal fluid without the need for multiple ventricular or lumbar punctures. Additionally, it facilitates the convenient intraventricular administration of medications to control intracranial infections and promote hematoma absorption.
This article categorizes the common chief complaints of patients into four main groups. The first category comprises manifestations of intracranial hypertension, including headaches, vomiting, somnolence, and fontanelle hypertension. The second category involves leakage of the shunt system, which had been placed in the first operation, either at the scalp or anal region. The third category includes abdominal pain, and the last one is fever. Following the identification of these four major categories of chief complaints, further investigations are conducted to confirm the underlying causes. This process involves the utilization of medical history, radiology examination, serological analyses, and microbiological examinations. The objective of all these auxiliary investigations is to ascertain the underlying etiology, thereby determining the presence of high-risk factors for complex hydrocephalus and assessing the likelihood of its occurrence. Subsequently, a two-stage surgical plan is tailored based on the patient's individual circumstances, such as overall health status and personal preferences, significantly improving the prognosis for complex hydrocephalus.
If there are manifestations of intracranial hypertension, it may indicate progressive enlargement of the ventricular system primarily or inadequate drainage by the shunt system used in previous shunt surgery, which can be discriminated by medical history and radiology examination. Primary hydrocephalus can be confirmed through radiology examinations, distinguishing between congenital hydrocephalus (commonly due to developmental abnormalities of the ventricular system) and secondary hydrocephalus resulting from intracranial hemorrhage. In cases of congenital hydrocephalus where the etiology is clear and the condition is straightforward, if the patient is a term infant with normal birth weight, symptoms can often be effectively alleviated through standard shunt surgery, leading to gradual normalization of the ventricular system over time (21). However, if the patient is a preterm infant with very low birth weight, it will become complex hydrocephalus, which requires EVD with an Ommaya reservoir first, followed by VPS (22). Meanwhile, hydrocephalus secondary to intracranial hemorrhage is not only a contraindication for routine shunt surgery but also represents a form of complex hydrocephalus. Additionally, the presence of intraventricular blood can lead to the formation of fibrous septa within the ventricular system, further complicating the condition into multiloculated hydrocephalus (23).
If malfunction of the previous shunt system is identified, indicating initial surgical failure and meeting the definition of complex hydrocephalus, it is crucial to assess for any signs of infection through temperature measurement, white blood cell count, C-reactive protein, erythrocyte sedimentation rate, and CSF culture. The above tests are also required in the conditions mentioned previously, where the second category involves leakage of the shunt system and the third indicates abdominal pain. If there is no infection, it will be considered simple hydrocephalus that can be resolved by replacing the shunt system, placing the original shunt system following disinfection, or altering to a VAS. However, in cases of intracranial infection, it will be classified as complex hydrocephalus, necessitating the immediate removal of the shunt, followed by appropriate management of the infection.
If a patient presents with fever along with changes in the characteristics of CSF, broad-spectrum antimicrobial therapy should be initiated alongside serological and microbiological examinations to identify the pathogen. Once the pathogen is identified, targeted antimicrobial therapy should be administered. It is essential to differentiate between different pathogens to determine the sequence of treatment modalities. For pathogens such as Mycobacterium tuberculosis (24), Cryptococcus neoformans (25), or Candida albicans (26), which are considered specific types of intracranial infections, the treatment approach differs. The significant goal in these cases is to reduce intracranial pressure through shunt surgery, followed by anti-infection treatment. If the infection is caused by common gram-positive or gram-negative bacteria, it is considered a standard infection. Infection is a contraindication for routine shunt surgery and represents a form of complex hydrocephalus. Similarly, due to the action of microorganisms within the skull, an immune response is triggered (27), leading to the proliferation of substances like collagen and the formation of septa within the ventricular system, resulting in multiloculated hydrocephalus. Existing studies mostly regard infection as a postoperative complication of hydrocephalus, but less attention is paid to infection as a major factor of complex hydrocephalus (28). In this paper, it is believed that for hydrocephalus post-infection, the most important step is to control the infection until the cerebrospinal fluid is clear and free of pathogenic microorganisms, after which surgery can be carried out.
In cases where intracranial hemorrhage or infection is suspected, radiology examinations are crucial to assess the presence of septations within the ventricular system and the circulation of cerebrospinal fluid, aiding in the diagnosis of complex multiloculated hydrocephalus (29). If multiloculated hydrocephalus is confirmed, the initial preparatory step involves endoscopic fenestration of the cysts to establish communication between them, converting multiloculated hydrocephalus into uniloculated hydrocephalus. This reduces the number of required shunt catheters and minimizes the need for multiple shunting procedures. Subsequently, EVD is used to temporarily drain CSF. Existing research mostly focuses on comparing the advantages and disadvantages of craniotomy and endoscopic surgery, which is of certain significance (30). Through such research, medical institutions can learn and master relevant surgical skills accordingly, and also guide doctors' choice of surgical methods to a certain extent. However, this study holds that compared with the choice of surgical form, for multiloculated hydrocephalus, the most important step is to overcome the obstacles and establish communication first, rather than adhering strictly to a particular surgical form.
For patients with uniloculated hydrocephalus, the first stage involves EVD and placement of an Ommaya reservoir. This allows intermittent drainage of CSF to promote hematoma resolution and facilitates intraventricular administration of antimicrobial agents (31). After the first stage of surgery, anti-infection treatment is continued until CSF routine examination and biochemical tests confirm that the CSF is clear and sterile (32), indicating successful control of intracranial hemorrhage and infection. The patient can then proceed to the second stage of formal surgery.
At this point, the patient often no longer has contraindications to shunt surgery, and the likelihood of developing complex hydrocephalus is significantly reduced. Based on the patient's current overall health status, VPS should be the preferred choice if there are no abdominal or peritoneal diseases; otherwise, VAS is preferred. Patients typically experience symptom relief shortly after formal surgery, and regular follow-up examinations post-discharge will show gradual normalization of the ventricular system.
In cases of severe and refractory hydrocephalus with special circumstances, which match the first definition of complex hydrocephalus mentioned above, it is necessary to consider the procedure of surgical management for complex hydrocephalus again. Alternative surgical approaches should be considered because VPS or VAS may be ineffective for that patient. These may include ETV to establish new intracranial circulation or CPC to reduce cerebrospinal fluid secretion.
In addition to the scenarios mentioned above, there is another significant category of complex hydrocephalus cases involving preterm newborns. Preterm newborns often have very low birth weights and poor overall health, making them intolerant to surgery. Even if surgery is attempted, the outcomes are usually poor, leading to prolonged non-recovery, repeated surgeries, and significant physical, psychological, and financial burdens for the patients. Additionally, they are often associated with intracranial hemorrhage and are more susceptible to intracranial infections due to their weakened immune systems, representing two or more types of complex hydrocephalus. Thus, whether the infant has intracranial hemorrhage and infections or is diagnosed with congenital hydrocephalus, it is undeniable that preterm newborns should be managed according to the complex hydrocephalus protocol.
To address this, we typically perform a two-stage approach: In the first stage, EVD with Ommaya reservoir implantation is carried out. This approach allows us to control the existing intracranial hemorrhage and infection while delaying the need for further interventions. During this time, the infant can continue to develop in a relatively stable environment. Once the infant reaches a postmenstrual age greater than 37 weeks and a weight exceeding 2.5 kilograms, their overall health status becomes more tolerant of surgery. At this point, the second stage involves VPS. For these infants, this treatment approach yields more significant therapeutic effects and a better prognosis.
In clinical practice, clinicians should carefully understand the process, starting from the initial diagnostic symptoms, and use various auxiliary examination methods to clarify the real situation of patients. They should assess whether there are factors that may lead to complex hydrocephalus, prevent problems before they occur, and prepare in advance for diagnosis and treatment according to the two-stage surgical procedure. Based on the analysis of typical cases in this study, it is evident that all three patients presented with two or more high-risk factors for complex hydrocephalus during their initial consultations. It can be almost conclusively determined that these patients did not have simple hydrocephalus, and the likelihood of successful cure through a single surgery was minimal.
For the first two cases, we adopted the procedure of surgical management for complex hydrocephalus proposed in our new summary: EVD with Ommaya reservoir implantation to control existing intracranial hemorrhage and infection in the first stage. After confirming that the cerebrospinal fluid is clear, the hematoma is absorbed, and the infection is controlled, the second stage involved VPS placement. Symptoms were alleviated immediately postoperatively, and subsequent imaging results showed normalization of the ventricular system.
Since complex hydrocephalus is a chronic condition, a discussion on long-term complications will be beneficial. From our results, patients who underwent the two different procedures of surgical management showed almost no difference in subsequent symptom relief, normal imaging ventricles, and survival rate. This also indicates that for complex hydrocephalus, unless individual patients have special circumstances, there is generally no difference in the cure of the disease. However, inappropriate surgical procedures will greatly increase the number of operations undergone by patients, especially for children, who are in a critical period of growth and development. The impact of surgery can affect their subsequent cognition, development, and function, which is also reflected in the results of this article. In addition to these injuries, continuous surgery affects the quality of life and economic situation of patients, bringing both physical and psychological distress.
Moreover, long-term complications are a major factor affecting patients' lives. Slit ventricle syndrome is mainly due to the siphoning effect of the shunt tube, which leads to excessive shunting of cerebrospinal fluid. Eventually, the ventricle becomes narrow and slit-like, requiring reoperation to replace the shunt device (33). Shunt dependency syndrome often arises due to the long-term placement of the shunt, decreased brain tissue compliance, and increased intracranial pressure from a small amount of cerebrospinal fluid, causing headaches. It is often difficult to remove the shunt and switch to ETV (34). Sylvian Aqueduct Syndrome often compresses the brain tissue due to the reversal of supratentorial and infratentorial pressure after shunt operation, resulting in ocular symptoms, and ETV is needed again to balance the pressure (35).
Of course, we also acknowledge that this study has its limitations. The patients selected in this paper are all from Xiangya Hospital, making it a single-center study. The temporal and regional characteristics of the sample sources have not been further analyzed, and they do not have a wider range of universality. Additionally, it is a retrospective study, so it is not random in patient selection, and some selection bias may occur. To conduct better research, we plan to collaborate with several affiliated units to collect samples for analysis in future work. Simultaneously, randomized controlled trials will be gradually carried out after careful design of the research protocol.
In summary, through meticulous review of the literature and consolidation of clinical cases within our institution, we have pioneered a relatively comprehensive and systematic procedure of surgical management for pediatric complex hydrocephalus. This protocol encompasses early identification and diagnosis, followed by personalized therapeutic interventions tailored to the etiology and individual patient circumstances, implemented in two stages. By intervening early and effectively, we have succeeded in curtailing the progression of complex hydrocephalus, thereby enhancing the therapeutic experience and quality of life for our patients.
5.1. Conclusions
(1) Complex hydrocephalus is defined as hydrocephalus requiring multiple surgeries for cure, caused by hemorrhage and/or infection, multiloculated hydrocephalus, and preterm infants with very low birth weight.
(2) The treatment principle lies in early identification for prompt diagnosis and timely staged treatment in both early and late phases. Early comprehensive treatment aims to prevent progression to complex hydrocephalus, with personalized interventions tailored accordingly.
(3) In the early first stage, EVD with Ommaya reservoir implantation is performed. This aims to control pre-existing intracranial hemorrhage and intracranial infection while allowing preterm infants to gain developmental time. Endoscopic cyst fenestration converts multiloculated hydrocephalus into uniloculated hydrocephalus.
(4) There is no absolute preference among various surgical techniques in the subsequent second stage. The choice depends on the patient's condition, sometimes necessitating sequential usage to manage extremely refractory hydrocephalus.
(5) Postoperative follow-up is essential, with regular imaging examinations to assess the restoration of the ventricular system. Timely identification and implementation of new interventions are crucial in managing any evolving complications.