1. Background
Community-acquired pneumonia (CAP) in childhood is an acute infection of the lung parenchyma that is caused by a pathogen outside the hospital, i.e., in the community. This disease is one of the most important causes of illness in developed countries and one of the leading causes of death in developing countries (1). According to the World Health Organization (WHO), approximately 2 million children under 5 years die from pneumonia worldwide, with most of them occurring in developing countries. The mortality rate in developed countries is less than 1 in 1,000 people per year. The frequency of CAP diagnosis is about 10 times higher in the developing world where, globally, there are over 1,400 cases of CAP per 100,000 children every year, with the greatest incidence occurring in South Asia (2,500 cases per 100,000 children) and West and Central Africa (1,620 cases per 100,000 children). Fortunately, due to the high level of the health-care systems, mortality is relatively low in industrialized countries. On the contrary, in the developing world, CAP continues to kill over 800,000 children under 5 every year, or around 2,200 every day (2). In general, CAP is associated with significant costs, including direct medical expenses and indirect costs from lost work hours by parents of involved children (3). In developed countries, mortality from CAP has decreased significantly over the past decades, but recently, an increase in the incidence of pulmonary complications of CAP has been reported. These complications include parapneumonic effusion (PPE), pleural empyema (PE), Necrotizing pneumonia (NP), and Lung abscess (4). While PPE is the most common pulmonary complication of CAP, NP is the most severe condition associated with prolonged hospitalization and antibiotic therapy in these patients. According to a recent audit by the British Thoracic Society on pediatric pneumonia, conducted from November 1, 2016, to January 31, 2017, in the UK, complicated CAP accounted for approximately 3% of all cases (1% PE, 1% PPE, 0.3% Lung abscess, lower values for NP) (5).
In some patients, signs and symptoms associated with pulmonary complications may be the initial manifestation of CAP (6). However, in most children, these complications develop in the later stages of the disease and are not present at first. Interestingly, the increased incidence of pulmonary complications is observed not only in untreated or inadequately treated children but also in patients treated according to current guidelines. The reasons for this phenomenon in patients with CAP are still unclear, and conflicting findings exist. Therefore, this study aims to evaluate the factors that may be effective in predicting progression to pulmonary complications in children with CAP.
1.1. Key Points
(1) As a common disease in children living in developing countries, CAP causes many problems for patients and their families. Identifying the risk factors for local complications in these patients can help prevent them. This, in turn, will improve the quality of services provided to these patients and effectively reduce the time and cost burden on their families.
(2) Improving the quality of services provided to patients with CAP and reducing the complications caused by this disease in affected children can significantly reduce the costs imposed on society and industry. Furthermore, better outcomes from improved patient management can contribute to the advancement of the medical industry.
2. Methods
We included all children hospitalized in Zahra Mardani Azari Children's Hospital in Tabriz due to CAP between October 2022 and October 2023. The sample size was determined using the following formula [n' = n/ (1 (z2 × p̂ (1-p̂))/ (ε2 × N))]: Z represents the z score, ε is the margin of error, N is the size of the target population, and p is the desired prevalence in the population. Based on the results of Land et al.'s investigation (7), which reported a 27.9% prevalence of local complications following CAP in children, and considering a 95% confidence interval and 5% alpha error, the minimum total sample size required for the study was calculated to be 153 patients. One of the main criteria for inclusion of patients is complete data records; so, in the case of missing variables, we excluded the patient.
The study's inclusion criteria involved children hospitalized due to CAP and between the ages of two months and 18 years. Patients under two months old or over 18 years old, those with incomplete file information, those suffering from hospital-acquired pneumonia, and with underlying chronic lung diseases such as cystic fibrosis, congenital lung diseases (chronic lung disease or other congenital airway anomalies), or bronchiectasis were excluded from the study. Additionally, patients at an increased risk of aspiration due to conditions such as neurological disease or swallowing disorders and those with primary and secondary immunodeficiency were also excluded.
Patients were grouped based on the clinical course of the disease, including: (1) those with Para-Pneumonic Effusion/Pleural Empyema (PPE/PE), Necrotizing Pneumonia (NP), or Lung abscess; and (2) those without pulmonary complications.
Finally, the patients in the two groups were compared in terms of demographic information (including: Age, sex, weight, height, and BMI); prescription records (including: The history of antibiotics, antipyretic treatment including acetaminophen and ibuprofen, and anti-inflammatory treatments); medicines prescribed during hospitalization (including: Antipyretics, anti-inflammatories, and antibiotics, etc.); clinical signs and symptoms (including: Fever, respiratory rate, and cough); laboratory findings at admission and during hospital treatment including [CBC (Complete Blood Count), ESR (Erythrocyte Sedimentation Ratio), CRP (C-Reactive Protein), Bun (Blood Urea Nitrogen), Cr (Creatinine), VBG (Venous Blood Gases)]; imaging results [Chest X-ray and CT-Scan (Computed Tomography Scan)]; and the clinical course of the disease [including the time of onset of clinical symptoms and the time of onset of Fever until hospitalization].
The study data was analyzed using SPSS (version 23.0 for Windows, SPSS Inc., Chicago, IL, USA) software. Using a frequency table, quantitative data were presented as either mean ± SD or median with interquartile range (IQR). Qualitative data were shown as frequency (%). Data were compared by independent t-test and chi-Square test. To identify the risk factors for pulmonary complications in children with pneumonia, we used univariate and multivariate logistic regression analysis and presented the results using odds ratio (OR) with a 95% confidence interval. A P-value less than 0.05 was considered significant.
The study protocol adhered to the ethical guidelines of the 1975 Declaration of Helsinki. This study was approved by the Research Ethics Committee of Local Tabriz University of Medical Sciences (IR.TBZMED.REC.1402.569).
3. Results
Three hundred sixty-one patients were examined, with 104 (28.8%) in the case group and 257 (71.2%) in the control group. The frequency of Pulmonary complications including: Parapneumonic effusion, empyema, lung abscess, and necrotizing pneumonia were 85 (81.7%), 21 (20.2%), 7 (6.7%), and 29 (27.9%); respectively. Table 1 compares the demographic information of patients.
| Variables | Case (n = 104) | Control (n = 257) | P-Value |
|---|---|---|---|
| Male/female | 49 (47.1) / 55 (52.9) | 140 (54.5) / 117 (45.5) | 0.125 |
| Age (mo) | 63.89 ± 29.50 (median = 66) | 44.65 ± 34.02 (median = 40) | 0.018 |
| Weight (kg) | 21.82 ± 10.63 (median = 19.25) | 15.90 ± 8.57 (median = 14) | 0.001 |
| Height (cm) | 111.54 ± 19.85 (median = 110) | 96.07 ± 22.98 (median = 98) | 0.006 |
| BMI (kg/m2) | 17.92 ± 11.89 (median = 16) | 16.03 ± 3.17 (median = 15.5) | 0.136 |
Abbreviations: kg, Kilogram; cm, centimeters; BMI, Body Mass Index; m, meter.
a Data were presented in frequency (%) or mean ± SD (median).
b P-value < 0.005 is considered as significant.
The comparison of the frequency of different types of medication received by children in the groups is detailed in Table 2. The findings show that in the case group, the rate of receiving oral acetaminophen (13.5% vs. 6.6%; P = 0.032) and IV acetaminophen (63.5% vs. 33.9%; P = 0.001) was significantly higher than in the control group.
| Variables | Case (n = 104) | Control (n = 257) | P-Value |
|---|---|---|---|
| Anti-pyretic | |||
| Acetaminophen oral. | 14 (13.5) | 17 (6.6) | 0.032 |
| Acetaminophen IV. | 66 (63.5) | 87 (33.9) | 0.001 |
| Acetaminophen Supp. | 0 | 1 (0.4) | 0.712 |
| Ibuprofen | 1 (1) | 0 | 0.288 |
| Anti-inflammatory drugs | |||
| Methylprednisolone (normal dose) | 18 (17.3) | 27 (10.5) | 0.058 |
| Methylprednisolone (pulse dose) | 4 (3.8) | 0 | 0.007 |
| Naproxen | 0 | 1 (0.4) | 0.712 |
| Hydrocortisone | 3 (2.9) | 4 (1.6) | 0.325 |
| Dexamethasone | 1 (1) | 0 | 0.288 |
| Prednisolone | 1 (1) | 0 | 0.288 |
| Oseltamivir | 64 (61.5) | 163 (63.4) | 0.413 |
| Antibiotics | |||
| Co-Amoxiclav | 1 (1) | 0 | 0.288 |
| Ampicillin | 0 | 2 (0.8) | 0.506 |
| Ceftazidime | 4 (3.8) | 3 (1.2) | 0.109 |
| Cefotaxime | 9 (8.7) | 34 (13.2) | 0.150 |
| Ceftriaxone | 78 (75) | 218 (84.8) | 0.034 |
| Clindamycin | 35 (33.7) | 81 (31.5) | 0.329 |
| Imipenem | 1 (1) | 0 | 0.288 |
| Meropenem | 63 (60.6) | 84 (32.7) | 0.001 |
| Vancomycin | 87 (83.7) | 87 (33.9) | 0.001 |
| Azithromycin | 2 (1.9) | 12 (4.7) | 0.180 |
| Clarithromycin | 0 | 1 (0.4) | 0.712 |
| Amikacin | 8 (7.7) | 2 (0.8) | 0.001 |
| Linezolid | 4 (3.8) | 0 | 0.007 |
| Ciprofloxacin | 1 (1) | 0 | 0.288 |
| Fluconazole | 1 (1) | 0 | 0.288 |
a Data were presented in frequency (%).
b P-value < 0.005 is considered as significant.
The clinical findings, including body temperature, respiratory rate, and presence of cough, are presented in Table 3 (Full Para-clinic findings were represented in Appendix 1 in Supplementary File). In the control group, one patient was intubated after hospitalization. The results indicated a significantly higher mean number of breaths (43 breaths per minute vs. 35 breaths per minute; P = 0.001) in the patients of the case group compared to the control group. Nonetheless, the mean time interval between the onset of fever and the time of visiting was significantly longer in the case group compared to the control group (7 days vs. 5 days; P = 0.003). Regarding the chest X-ray, the results showed that there is a significant frequency of lung involvement in the right middle (1.9% vs. 10.5%; P = 0.003), left upper (1% vs. 7.4%; P = 0.009), and left lower lung lobes (1% vs. 7.4%; P = 0.009) in the case group, which is less than the control group. Regarding the CT-Scan, it was also observed that the frequency of lung involvement in the right upper (46.2% vs. 29.6%; P = 0.002), right lower (54.8% vs. 24.5%; P = 0.001), left upper (32.7% vs. 13.6%; P = 0.001), and left lower lobes (53.8% vs. 35.8%; P = 0.001) in the case group was more than the control group. Meanwhile, the frequency of positive HRAD cases in the case group was significantly lower than in the control group (4.8% vs. 13.2%; P = 0.012). Complete imaging findings were represented in Appendix 2 in Supplementary File.
| Variables | Case (n = 104) | Control (n = 257) | P-Value |
|---|---|---|---|
| Body temperature | 38.52 ± 0.71, (median = 38.60) | 38.23 ± 0.81, (median = 38.50) | 0.719 |
| Respiratory rate | 44.67 ± 12.98, (median = 43) | 39.62 ± 12.83, (median = 35) | 0.001 |
| Abnormal c | 96 (92.3) | 201 (78.2) | 0.001 |
| Cough | 104 (100) | 256 (99.6) | 0.712 |
| Time from onset of symptoms to visit (day) | 8.72 ± 5.21, (median = 7) | 7.95 ± 5.84, (median = 7) | 0.255 |
| Time from onset of fever to visit (day) | 7.27 ± 4.88, (median = 7) | 5.60 ± 4.30, (median = 5) | 0.003 |
aData were presented in frequency (%).
b P-value < 0.005 is considered as significant.
c Abnormal cases are determined based on the standard range of the patient's age.
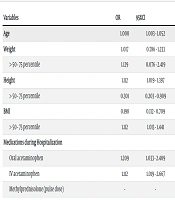
The results of the univariate logistic regression analysis for predicting Pulmonary complications in CAP patients are presented in Table 4. The risk factors examined included weight (OR = 1.129), height (OR = 1.112), BMI (OR = 1.112), administration of oral and intravenous acetaminophen during hospitalization (OR = 1.112, 1.209; respectively), tachypnea (OR = 5.178), duration of fever (OR = 1.290), ESR (OR = 1.312), and HRAD (OR = 3.473) during hospitalization.
| Variables | OR | 95%CI | P-Value |
|---|---|---|---|
| Age | 1.000 | 1.003 - 1.052 | 0.146 |
| Weight | 1.017 | 0.786 - 1.233 | 0.313 |
| > 50 - 75 percentile | 1.129 | 0.076 - 2.419 | 0.045 |
| Height | 1.112 | 1.019 - 1.397 | 0.001 |
| > 50 - 75 percentile | 0.301 | 0.203 - 0.909 | 0.665 |
| BMI | 0.198 | 0.112 - 0.709 | 0.893 |
| > 50 - 75 percentile | 1.112 | 1.013 - 1.441 | 0.003 |
| Medications during Hospitalization | |||
| Oral acetaminophen | 1.209 | 1.033 - 2.409 | 0.003 |
| IV acetaminophen | 1.112 | 1.019 - 2.667 | 0.002 |
| Methylprednisolone (pulse dose) | - | - | 0.999 |
| Clinical Course | |||
| Tachypnea | 5.178 | 2.233 - 8.112 | 0.001 |
| Fever onset time | 1.290 | 1.101 - 2.023 | 0.002 |
| Para-Clinics (by Abnormal Tests) | |||
| WBC | 1.890 | 1.403 - 2.210 | 0.001 |
| Neutrophil | 0.889 | 0.779 - 1.014 | 0.081 |
| Lymphocyte | 0.931 | 0.802 - 1.081 | 0.350 |
| ESR | 1.312 | 0.938 - 2.409 | 0.001 |
| BUN | 0.942 | 0.877 - 1.012 | 0.105 |
| Imaging Involvement (CT-Scan) | |||
| Right upper lobe | 0.598 | 0.354 - 1.010 | 0.197 |
| Right lower lobe | 0.302 | 0.182 - 0.501 | 0.201 |
| Left upper lobe | 0.534 | 0.292 - 0.980 | 0.214 |
| Left lower lobe | 0.580 | 0.350 - 0.960 | 0.229 |
| HRAD (Neg) | 3.473 | 1.244 - 9.969 | 0.017 |
Abbreviations: OR, odds ratio; CI, confidence interval.
Multivariate regression results (Table 5) showed that height (OR = 1.314), administration of oral acetaminophen (OR = 1.323), tachypnea (OR = 7.178), fever onset time (OR = 2.554), and negative HRAD imaging results (when there is not any pathology seen in imaging evaluation) (OR = 4.090) were significant predictors of complicated CAP.
| Variables | OR | 95%CI | P-Value |
|---|---|---|---|
| Demographics | |||
| Age | - | - | 0.706 |
| Weight | - | - | 0.223 |
| Height | 1.314 | 0.998-1.677 | < 0.001 |
| BMI | - | - | 0.404 |
| Medications during hospitalization | |||
| Oral acetaminophen | 1.323 | 1.011-2.758 | < 0.001 |
| IV acetaminophen | - | -- | 0.719 |
| Clinical course | |||
| Tachypnea | 7.178 | 1.033-11.098 | < 0.001 |
| Fever onset time | 2.554 | 1.403-5.023 | < 0.001 |
| Imaging involvement (CT-Scan) | |||
| Right upper lobe | - | - | 0.550 |
| Right lower lobe | - | - | 0.474 |
| Left upper lobe | - | - | 0.309 |
| Left lower lobe | - | - | 0.714 |
| HRAD (Neg) | 4.090 | 1.443-15.969 | 0.011 |
Abbreviations: OR, odds ratio; CI, confidence interval.
4. Discussion
We evaluated the factors associated with complications in pediatric patients hospitalized due to CAP. So, we included children admitted to Zahra Mardani Azari Children's Hospital in Tabriz, with CAP diagnosis between October 2022 and October 2023. Our evaluation of demographic findings revealed that patients with complicated CAP had significantly higher age, weight, and height than those without complications. This is consistent with Tan et al.'s (8) investigation, which found that children with complicated pneumonia were significantly older (45 months vs. 27 months). Additionally, a study by Masarweh et al. (9) in 2021 reported that increasing age was a risk factor for complicated CAP in children with OR = 1.131. A possible explanation is that younger children with pneumonia may be hospitalized even with a mild clinical course.
Our study compared the drug treatments received by CAP cases, and there was a statistically significant difference in the use of antipyretic drugs, anti-inflammatory drugs, and antibiotics between the two groups. The logistic regression results suggested that oral and intravenous acetaminophen received during hospitalization in children with pneumonia were predictive factors for CAP complications. It is important to note that some antibiotics were added to the patient's treatment regimen based on clinical evaluation, such as after CT-Scan evaluation and observation of complications, and these medications are not among the influential factors in the prognosis of patients. Unlike our results, Huang et al. (10) did not report a difference in antimicrobial treatment between complicated and uncomplicated cases. The disease's progress seems to depend on various factors, among which we can mention drug resistance and the bioavailability of drugs based on the factory and place of production. Hence, physicians follow clinical guidelines when prescribing antibiotics and drug treatment (3, 11, 12).
Our results showed that the respiratory rate and the average duration of fever onset in complicated CAP cases were significantly higher than in uncomplicated cases (OR = 5.178, 1.290; respectively). Consistent with our study, Wexler et al. (13) reported that patients with complicated CAP had a longer length of hospitalization (13.2 days vs. 8.3 days) and duration of fever (9.2 days vs. 5.1 days). Also, Chalmers et al. (14) reported a result consistent with our study. The available evidence has shown that persistent fever for more than 72 hours after hospitalization is one of the essential clinical factors associated with aggravated pneumonia (11, 15).
We found that WBC counts above 13,750/µL (OR = 1.890) and ESR above 80 mm/hr (OR = 1.312) are predictors of complicated CAP. Elmeazawy et al. (16) reported that low levels of MPV and high D-dimer are potential predictors of complicated CAP in children with pneumonia (15, 17, 18). Additionally, evidence suggests that high CRP and ESR levels at admission may indicate an increased risk of parapneumonic effusion or empyema in pediatric pneumonia cases. Previous studies have shown that leukocytosis, especially a WBC count greater than 15,000/µL, is directly correlated with severe pneumococcal disease and can help differentiate between viral and bacterial pneumonia (19-23).
The evaluation of imaging findings in our patients revealed that the frequency of abnormal cases in chest X-rays and CT scans for complicated pediatric cases is significantly lower than in patients without complications. Also, a negative result of HRAD before hospitalization (OR = 3.473) is a predictive imaging factor for complicated CAP. Prior studies have also indicated that observing chest involvement at the beginning of hospitalization can lead to better patient prognosis (24). For patients with CAP with a typical chest X-ray result (any sign of lung involvement in any lobes, such as consolidation, etc.) at the beginning of hospitalization, other influencing factors should be considered so that cases with a high risk of complications receive prompt treatment.
Complications from pneumonia can occur even when children with the disease are hospitalized and treated. These complications include pleural or parapneumonic effusion, pneumothorax, acute respiratory distress, empyema, necrotizing pneumonia, bronchopulmonary fistula, pneumatocele formation, and lung abscess. Such complications can lead to prolonged hospitalization, the need for surgery, and irreversible consequences in pediatrics (3). In the study, parapneumonic effusion was the most common pulmonary complication, occurring in 81.7% of cases. Other complications, in order of frequency, included necrotizing pneumonia (27.9%), empyema (20.2%), and lung abscess (6.7%). No cases of mortality were observed in the study.
The study's main limitation is the lack of information about antibiotic resistance and sensitivity, as well as the strains isolated from patients. This is a single-center study, which may limit the generalizability of the results. Additionally, due to the retrospective design, there are limitations regarding some potential confounding factors.
We concluded that the incidence rate of complicated CAP cases in Zahra Mardani Azari Children's Hospital in Tabriz in 2023 was 28.8%. High weight and BMI, receiving acetaminophen during hospitalization, tachypnea, duration of fever until admission, and high WBC and ESR are predictors of pulmonary complications in children with CAP. It is recommended to use these factors in evaluating patients during the initial pediatrician clinical visits to minimize the risk of complications in CAP patients. In future research, we propose investigating the impact of inflammatory factors that can be evaluated by a simple complete blood count test, such as MPV (mean platelet volume), NLR (neutrophil to lymphocyte ratio), and PLR (platelet to lymphocyte ratio), in complicated CAP cases.