1. Background
Zinc (Zn) is an essential micronutrient that plays an important role at different molecular and cellular levels. This element is involved in protein synthesis, gene expression, cellular metabolism, nuclear division, signal transduction, hormonal structure, and enzymatic function (1-3). Despite its abundant functions, the level of Zn is constantly maintained within narrow biological limits (4, 5). Studies have shown the role of Zn in fetal and neonatal growth and development (6). Maternal blood Zn levels decrease until 35 weeks of gestation due to increased blood volume, organ metabolism, and urinary excretion, as well as hormonal alterations and active maternal-placental transport. In addition to inadequate Zn intake, perinatal co-morbidities, such as digestive complications, may put pregnant women at a higher risk of Zn deficiency (6). Previous studies have pointed to the possible teratogenic effects related to Zn deficiency, which can result in adverse pregnancy outcomes (7, 8). Preterm birth, preterm premature rupture of membranes, preeclampsia, small for gestational age, intrauterine growth restriction, and low Apgar scores are several reported maternal and neonatal morbidities associated with prenatal Zn deficiency (8, 9).
Evidence has also shown low serum Zn concentrations in neonates with higher weight gain due to increased cellular metabolism and enzymatic activities. Accordingly, Zn deficiency may result in delayed neonatal growth (10). It has also been demonstrated that Zn intake during the first 12 months could affect growth outcomes in both term and preterm infants (11). As Zn deficiency may limit infant growth, this diagnosis should be considered, particularly for premature infants with failure to thrive despite apparently receiving enough calories and macronutrients (12).
Concerning the relationship between Zn levels and anthropometric measures, Gomez et al. in 2015 showed a positive significant correlation between cord blood Zn levels and birth weight (Ρ = 0.283; P = 0.005) (13). Khoushabi et al. also demonstrated that of 60 included neonates, 12% were low birth weight, while the others had normal birth weight. The results of the study indicated that the mean Zn levels in the cord blood of neonates with normal birth weight (88.3 ± 12 mg/dL) were significantly higher (P < 0.05) than those of their low-birth-weight counterparts (70.0 ± 9.9 mg/dL) (14). This significant relationship between cord blood Zn levels and newborn birth weight was also confirmed by a systematic review and meta-analysis by Atazadegan et al. (15). Nanbakhsh and Tabrizi in 2017 demonstrated the effects of maternal serum Zn concentrations on neonatal birth weight. According to the findings of this study, mothers with serum Zn levels > 70 µg/dL had neonates with birth weights > 3500 g, while mothers with serum Zn levels < 60 µg/dL had neonates with birth weights < 3000 g. In addition, low birth weight neonates had significantly lower umbilical cord Zn levels than neonates with normal birth weights (19.86 ± 79.16 vs. 95.14 ± 17.56 µg/dL; P < 0.02) (16).
2. Objectives
Zinc deficiency is one of the most frequent deficiencies among women, particularly pregnant women in developing countries, including Iran (17, 18). Accordingly, the World Health Organization promotes prenatal micronutrient supplementation containing Zn for all women (19). Moreover, despite a few investigations that have assessed the correlations between cord blood Zn levels and anthropometric measures, the results are conflicting (13, 20-25). Hence, the present study was carried out to examine the potential influence of cord blood Zn on term neonates’ growth parameters.
3. Methods
A cross-sectional study was conducted at Yas Complex Hospital, affiliated with Tehran University of Medical Sciences (Tehran, Iran), in 2021. The study population included mothers and their singleton, term neonates (gestational age > 37 weeks). All mothers received supplements during their pregnancy. Written consent was obtained before the participants' enrollment. Exclusion criteria included multiple gestations, preterm birth, placental abnormalities, and a positive history of maternal complications such as severe anemia and chronic disease.
All demographic and clinical data related to the mothers and neonates were extracted from medical records and recorded in a checklist. Neonatal anthropometric values, including birth weight, height, and head circumference, were also determined using a digital weight scale (g) and measuring tape (cm), respectively.
Immediately after birth, blood sampling was performed, and 3 milliliters of umbilical cord blood were drawn, centrifuged, stored at -20°C, and sent to the hospital laboratory. Using the atomic absorption spectrophotometry method, Zn levels in cord blood (µg/dL) were measured (9, 26). Serum Zn levels below 65 µg/dL were considered deficient, while levels above 65 µg/dL were considered normal (21).
All gathered data related to Zn levels in cord blood, neonatal measurements, and anthropometric values were analyzed to determine possible correlations between Zn levels and neonatal anthropometric measures. Moreover, correlations between Zn levels and other variables were also assessed to identify any potential influencing factors.
3.1. Sample Size
Mohamed et al. in 2019 (24) demonstrated a Pearson correlation of 0.03 between Zn and weight. Using the following formula and considering a power of 80% and a confidence interval of 95%, the sample size was estimated to be 95.
r = 0.09; Z1-α/2 = 1.96; Z1-β = 0.84; n = 95.
3.2. Statistical Analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, USA), version 26.0. Descriptive data were presented in numbers (percent) and mean ± SD. The results of the non-parametric Kolmogorov-Smirnov test showed that the variables did not follow a normal distribution. Therefore, the Mann-Whitney and Pearson Correlation tests were used to analyze all data without normal distribution. P-values < 0.05 were considered significant.
3.3. Ethical Consideration
This study was approved in accordance with the Helsinki Declaration by the Ethics Board of Tehran University of Medical Sciences (ID Code: IR.TUMS.IKHC.REC.1400.282). Written consent was obtained from the included participants, no extra cost was imposed, and all data were treated as confidential.
4. Results
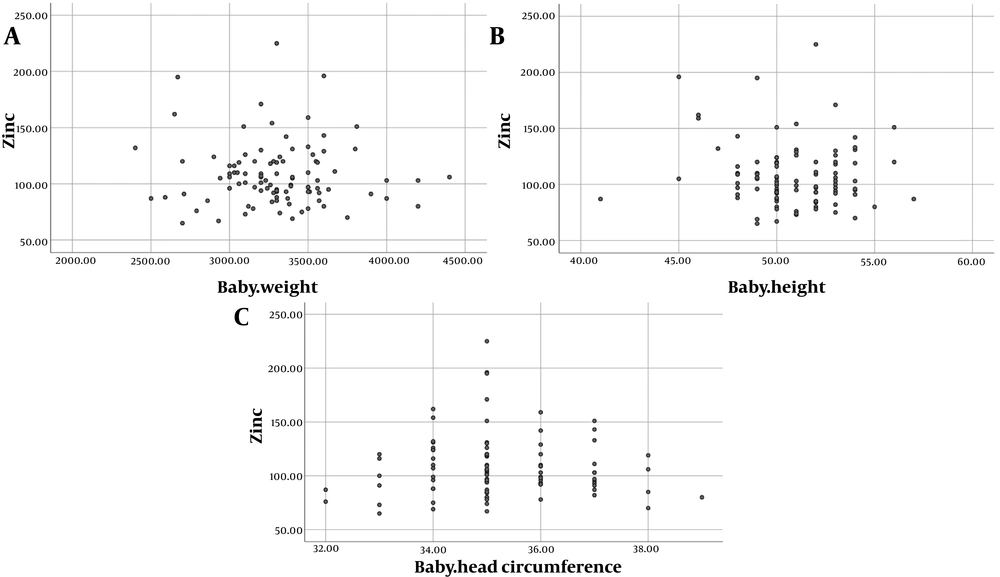
One hundred mothers with a mean age of 30.792 ± 5.908 years and a parity of 2.693 ± 1.181 were included. Sixty-eight of their neonates were male, and two newborns had low birth weight. The mean level of cord blood Zn was 107.703 ± 27.843 µg/dL (Min: 65 and Max: 225), and none of the neonates were Zn deficient. The mean Apgar scores at the first and fifth minutes, as well as birth weight, height, and head circumference, were 8.945 ± 0.271, 9.956 ± 0.253, 3301.020 ± 354.163 g, 50.816 ± 2.549 cm, and 35.319 ± 1.297 cm, respectively. The mean level of Zn in the neonates was 107.240 ± 27.590 (65.00 - 225.00) µg/dL. Figure 1 shows cord blood Zn levels in neonates with different birth weight, height, and head circumference parameters.
Evaluating the correlations between cord blood Zn levels and maternal and neonatal qualitative variables, data analysis showed no relationships between Zn levels and neonates’ sex (P = 0.70), low birth weight (P = 0.87), and maternal underlying disease (P = 0.49). Detailed data are shown in Table 1.
| Variables | Mean ± SD | P-Value |
|---|---|---|
| Neonate’s gender | 0.70 | |
| Male | 106.647 ± 27.100 | |
| Female | 108.500 ± 29.006 | |
| Low birth weight | 0.87 | |
| Yes | 109.500 ± 31.819 | |
| No | 108.010 ± 28.195 | |
| Maternal underlying disease | 0.49 | |
| Yes | 105.1064 ± 25.031 | |
| No | 109.9630 ± 30.129 |
Analysis of data regarding correlations between quantitative variables also showed no significant correlations between cord blood Zn levels and maternal age (P = 0.491), number of gravidity (P = 0.587), and neonates’ first (P = 0.118) and fifth minute (P = 0.207) Apgar scores. Neonates with higher anthropometric measures had lower cord blood Zn concentrations; however, the Zn values did not significantly alter neonates’ birth weight (P = 0.466), height (P = 0.466), and head circumference (P = 0.925). Cord blood Zn levels were also not correlated with blood pH (P = 0.532) or base excess (P = 0.068). Detailed data are shown in Table 2.
| Variables | Correlation Coefficient | P-Value |
|---|---|---|
| Maternal age | -0.069 | 0.491 |
| Gravidity | -0.055 | 0.587 |
| First minute Apgar score | -0.164 | 0.118 |
| Fifth minute Apgar score | -0.133 | 0.207 |
| Weight | -0.030 | 0.768 |
| Height | -0.074 | 0.466 |
| Head circumference | -0.010 | 0.925 |
| pH | -0.064 | 0.532 |
| Base excess | -0.185 | 0.068 |
5. Discussion
The present study aimed to assess the potential correlations between umbilical cord blood Zn concentrations and neonatal anthropometric parameters. Although the beneficial effects of micronutrients like Zn on pregnancy and neonatal outcomes have been reported by previous evidence (27-29), our results align with the results of a meta-analysis (19) suggesting that cord blood Zn levels may not exert a significant influence on neonatal growth outcomes.
The mean cord blood Zn level was found to be 107.7 µg/dL, with none of the neonates showing Zn deficiency, suggesting that maternal supplementation during pregnancy was effective in maintaining adequate Zn status in newborns. Fortunately, most pregnant women in our center received prenatal care from the first trimester of gestation. Supplements, including folic acid and iron, were administered to them based on the national protocol. In addition, during the second and third trimesters, pregnant women routinely took prenatal multivitamins containing vitamins and minerals. While this aligns with studies that emphasize the benefits of maternal Zn supplementation for fetal health (30, 31), the lack of a significant association between cord blood Zn and neonatal anthropometric measures may indicate that Zn’s role in fetal growth is complex. It is possible that Zn may play a more prominent role in postnatal growth and development (30), rather than exerting measurable effects on birth parameters. It also seems that larger sample sizes, through affecting variables, may influence the results.
The results of this study were in agreement with the results of Daniali et al. (22), who, through a cross-sectional study, evaluated the relationships between cord blood Zn concentrations and anthropometric measures in 226 neonates. The authors demonstrated no significant correlations between cord blood Zn levels and neonate height (P = 0.792), head circumference (P = 0.697), abdominal circumference (P = 0.785), chest circumference (P = 0.498), sex (P = 0.632), or Apgar score (P = 0.673). However, cord blood Zn level was significantly correlated with the neonate’s birth weight (β = 0.178; P = 0.02). In accordance with our findings, the results of a systematic review (32) have shown that maternal Zn supplementation did not significantly improve neonatal outcomes.
Amini et al. (23) demonstrated an inverse correlation between cord blood Zn levels and neonate birth weight (P = 0.008); however, no significant relationships were observed between blood Zn concentrations and other anthropometric measures like length or head circumference (P > 0.05). In contrast to our findings, several studies have shown diverse results. A systematic review (33) delineated a significant correlation between maternal Zn supplementation and neonate birth weight or length in 3 out of 9 included studies, while these findings were not confirmed by the other 6 studies. The results of a systematic review and meta-analysis (15) also demonstrated a significant correlation between cord blood Zn and neonate birth weight. Bayomy et al. (34) showed a significant and positive correlation between serum Zn levels and term neonate anthropometric measures, including weight, height, and head circumference.
Gomez et al. (13), in a case-control study, evaluated umbilical cord blood Zn levels in 123 neonates with different gestational ages and birth weights. The results of their study indicated significant correlations between umbilical cord Zn status and neonate birth weight and gestational age. Seriana et al. (35) showed significant relationships between Zn status at term pregnancy and the neonate’s height (P = 0.026) and head circumference (P = 0.012). On the other hand, this significant correlation was not observed with the neonate’s birth weight. Nanbakhsh and Tabrizi (16) also evaluated umbilical cord blood Zn levels in pregnant women. According to their findings, mothers with Zn levels > 70 µg/dL had neonates with birth weights > 3500 g, while mothers with Zn levels < 60 µg/dL had neonates with birth weights < 3000 g. Their results also showed that the mean Zn level in low-birth-weight neonates was significantly lower than in their normal-birth-weight counterparts.
These diverse results across studies might relate to differences in sample size, study design, including Zn assessment methods, study population, Zn metabolism, genetic factors, supplementation status, or participants’ gestational age. It is suggested that more investigations with larger sample sizes, considering more variables like prenatal supplementation status, maternal underlying diseases, perinatal complications, placental abnormalities, neonates’ gestational age, and health status, are needed.
According to the results, cord blood Zn level was not correlated with neonates' sex, Apgar scores, blood pH, or base excess. However, this also underscores the need for further research.
One of the confounding factors in the present study might be related to the use of supplements from different commercial brands. We assessed the available prenatal supplements in the market and found that the level of zinc was consistent (25 mg Zn). The study results should be interpreted with consideration of its limitations, such as the cross-sectional study design and relatively homogeneous population. Since non-supplemented mothers were not included in the study and the results showed no Zn-deficient mothers, we could not compare the neonates’ growth parameters in Zn-sufficient and Zn-deficient mothers.
Case-control studies with larger sample sizes and longitudinal follow-up are recommended to provide more informative insights into post-natal growth and development. Moreover, including multiple gestations and preterm neonates, as well as maternal lifestyle habits (nutritional and smoking status), biomarkers related to Zn metabolism, hematologic disorders like anemia, and placental complications, are factors that may influence the findings. Future investigations including such variables are suggested.
5.1. Conclusions
Although adequate Zn levels were observed in all neonates, these levels did not significantly influence neonatal anthropometric measures or immediate health indicators. Further studies with larger sample sizes are recommended to provide more comparative data, particularly regarding long-term growth outcomes.