1. Background
Acute bronchiolitis is the most common lower respiratory infection in young children and is the leading cause of hospitalization during this period (1). According to recent epidemiological data, the incidence of bronchiolitis has been increasing in recent years. Global warming and increased air pollution are thought to contribute to this rise (2).
Each year, bronchiolitis accounts for the hospitalization of 1 - 3.5% of healthy infants (3, 4). Although its incidence varies by season, it typically peaks in winter and early spring. Respiratory syncytial virus (RSV) is responsible for 50% of cases, while other related agents include parainfluenza virus, mycoplasma, and adenovirus (5). The pathophysiology of acute bronchiolitis is characterized by increased mucus production, mucosal and submucosal edema, peribronchial mononuclear cell infiltration, and obstruction of the small airways due to necrosis and shedding of epithelial cells (6). The clinical presentation of the disease can range from mild cases managed on an outpatient basis to severe cases requiring intensive care and mechanical ventilation.
Various scoring systems have been used to predict the prognosis of bronchiolitis, including the Wang score, respiratory distress assessment instrument (RDAI), and Respiratory Assessment Change Score (RACS). However, these systems have limitations. For example, they have been criticized for relying on subjective assessments and for not incorporating laboratory parameters (7). Therefore, there is a need for more objective and comprehensive prognostic tools.
The recently developed HALP score reflects the patient's overall well-being by combining various laboratory values. The HALP score is calculated using hemoglobin, albumin, lymphocyte, and platelet levels. The modified HALP (m-HALP) score is based on the product of these parameters and has been suggested to be more sensitive than the original HALP score (8). Previous studies have shown that this score, which includes albumin, hemoglobin, platelets, and lymphocytes, is a good predictor in patients with stomach, bladder, kidney, and prostate cancers, as well as in those with obesity and stroke (9-13). However, the use of HALP and m-HALP scores in acute infectious diseases is limited, and their effectiveness in pediatric acute respiratory infections, such as bronchiolitis, has yet to be investigated.
This score indicates the patient's systemic inflammatory status, overall nutritional status, and physiological well-being. Anemia and hypoalbuminemia are important signs of malnutrition in children. Lymphocytes play a major role in inflammation and are an important laboratory marker of the immune system's health. Platelet counts can increase or decrease in response to thromboembolism, hemorrhage, and sepsis (14). Low levels of albumin, hemoglobin, and lymphocytes, along with high platelet counts, have been associated with poor prognosis in various populations in previous HALP score studies.
Given this, we hypothesize that the HALP and m-HALP scores may play a role in assessing the prognosis of children with acute bronchiolitis and may predict the need for intensive care. Introducing this scoring system in bronchiolitis could provide a more objective and comprehensive evaluation of prognosis and disease progression.
2. Objectives
In the present study, we evaluated and compared the effectiveness of the HALP and the recently derived m-HALP scores in predicting the prognosis of children under 2 years old with acute bronchiolitis. This is the first time such a comparison has been made in the literature.
3. Methods
This study was conducted on 344 pediatric patients diagnosed with acute bronchiolitis between 2021 and 2023 in the pediatric clinic of a tertiary state hospital. The study was reviewed and approved by the hospital ethics committee (Decision Date: 25.01.2023, Decision Number: 2023/14). The diagnosis of acute bronchiolitis was based on the presence of clinical signs (cough, wheezing, tachypnea) and chest X-ray findings. The diagnosis was confirmed by two independent pediatricians. The study included patients under 2 years of age who were hospitalized and diagnosed with acute bronchiolitis after clinical and laboratory evaluation by a pediatrician.
The inclusion criteria were as follows: Age under 2 years, diagnosis of acute bronchiolitis, and hospitalization. The exclusion criteria were as follows: Missing laboratory data, referral to another center, attribution of dyspnea etiology to other causes, and presence of chronic lung disease or congenital heart disease. Data were obtained from hospital archive records and the computer system. Twenty-seven patients with missing laboratory data, those referred to an external center, and those for whom the etiology of dyspnea was attributed to other causes were excluded from the study.
Demographic data (age, gender, nutritional status), clinical characteristics (fever, respiratory rate, oxygen saturation), and duration of hospitalization were recorded. Laboratory measurements were performed using blood samples taken within the first 6 hours after the patients' initial admission to the hospital. Albumin, hemoglobin, platelet, lymphocyte, and neutrophil values were recorded from the blood samples collected at the time of the first admission.
These laboratory data were then used to determine the m-HALP score, HALP score, platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR). The equations used to calculate the HALP and m-HALP scores were albumin (g/L) × hemoglobin (g/L) × lymphocyte count (/L) / platelet count (/L), and albumin (g/L) × hemoglobin (g/L) × lymphocyte count (/L) × platelet count (/L), respectively.
The NLR was obtained by dividing neutrophil count by lymphocyte count, while the PLR was obtained by dividing platelet count by lymphocyte count.
According to the Wang bronchiolitis severity score, patients were scored based on respiratory rate (per minute), wheezing, retractions, general condition, and the presence of apnea. A score of 1 - 3 points was classified as mild, 4 - 8 as moderate, and 9 - 12 as severe apnea (15).
The primary outcome measure of the study was the need for intensive care unit (ICU) admission. Secondary outcome measures included the need for intubation and the length of hospital stay. Patients were followed up by telephone for 30 days after discharge, and any re-hospitalization was recorded.
HALP, m-HALP, NLR, and PLR values, as well as their relationships with the need for hospitalization in the ICU, need for endotracheal intubation, and the Wang bronchiolitis severity score, were evaluated.
Sample size calculation was based on previous similar studies. A total of 344 patients was determined to be sufficient, with 80% power and a 5% type I error rate. Potential confounding factors (age, gender, nutritional status) were controlled for by multivariate logistic regression analysis.
The chi-square test was used to determine whether there was a significant difference between the distribution of hospitalization status by gender. Independent samples t-tests were used to analyze whether laboratory variables, age, HALP, and m-HALP scores differed significantly according to hospitalization status. One-way ANOVA was used to test whether the scoring systems and NLR-PLR values differed significantly according to Wang score levels. Receiver operating characteristic (ROC) curve analysis was carried out to evaluate and compare the performance of the diagnostic markers. Receiver operating characteristic analysis was applied to HALP and m-HALP scores to predict ICU /ward hospitalization and intubation. The Youden J Index was used to determine the optimal cut-off value. Sensitivity, specificity, positive predictive value, and negative predictive value were obtained. Missing data were addressed using the multiple imputation method. A significance level of α = 0.05 was accepted. Statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corp., USA).
The study was conducted in accordance with the principles of the Declaration of Helsinki. Patient data confidentiality was maintained, and all data were anonymized. Written informed consent was obtained from the parents for participation in the study.
4. Results
The mean age of the 344 patients included in the study was 13.17 ± 9.28 months. When evaluating the nutritional status of the patients, 15% were underweight, 70% had normal weight, and 15% were overweight. The mean length of hospital stay was 5.3 ± 2.1 days.
The study included a total of 344 patients. Of these, 149 (43.31%) required ICU hospitalization. Among the patients hospitalized in the ward, 115 were female and 80 were male. Among those hospitalized in the ICU, 88 were female and 61 were male. There was no statistically significant difference between ICU and ward patients in terms of gender distribution (chi-square test, P = 0.98).
The mean HALP score of the patients hospitalized in the ward was 57.7, and the mean m-HALP score was 10 923 867.11. The mean HALP score of those hospitalized in the ICU was 49.49, and the mean m-HALP score was 7 716 345.12. When comparing the scoring systems, both were found to be significant in predicting the place of hospitalization (P = 0.00). An independent sample t-test was used for this comparison.
The main laboratory parameters were analyzed according to the place of hospitalization. There was a significant difference between ICU and ward patients in terms of NLR, PLR, albumin, lymphocyte, and neutrophil parameters. Hemoglobin (Hb) and platelet parameters were not significant. Independent sample t-tests were used for these analyses, and Bonferroni correction was applied. Table 1 shows these values.
| Variables | Ward (n = 195) | Intensive Care (n = 149) | P-Value |
|---|---|---|---|
| Age (mo) | 12.01 (9.48) | 14.68 (8.89) | 0.01 |
| Gender | 0.98 | ||
| Female | 115 | 88 | |
| Male | 80 | 61 | |
| Hb (g/L) | 111.66 (10.13) | 109.26 (13.04) | 0.05 |
| Albumin (g/L) | 43.11 (3.06) | 42.25 (3.93) | 0.02 |
| Lym (109/L) | 5.03 (2.22) | 4.11 (1.94) | 0.00 |
| Neu (109/L) | 5.52 (3.52) | 7.13 (4.07) | 0.00 |
| PLT (109/L) | 418.22 (115.45) | 397.69 (118.52) | 0.10 |
Comparison of Characteristics of the Patients in Terms of Intensive Care and Ward Hospitalization a
Physical examination findings of the patients were analyzed according to the Wang bronchiolitis severity score (Table 2). The mean values of the Wang score levels, along with the HALP and m-HALP scores, were determined, and it was analyzed whether they were correlated. Both HALP and m-HALP scores were found to be significant in determining the Wang score groups (mild-moderate-severe). One-way ANOVA was used for this analysis. Similarly, the mean values of NLR and PLR were determined for the Wang score groups (mild-moderate-severe), and changes in NLR and PLR values were found to be significant across the groups. One-way ANOVA was used for this analysis. Table 3 shows these results.
| Parameter | Ward (n = 195) | Intensive Care (n = 149) | Total (n = 344) |
|---|---|---|---|
| Respiratory rate | |||
| < 30 | 6 (3.1) | 0 (0) | 6 (1.7) |
| 30 - 45 | 120 (61.5) | 57 (38.3) | 177 (51.5) |
| 45 - 60 | 67 (34.4) | 89 (59.7) | 156 (45.3) |
| > 60 | 2 (1.0) | 3 (2.0) | 5 (1.5) |
| Wheezing | |||
| No | 38 (19.5) | 4 (2.7) | 42 (12.2) |
| Via stethoscope in expiration | 75 (38.5) | 28 (18.8) | 103 (29.9) |
| Via ear in expiration | 72 (36.9) | 75 (50.3) | 147 (42.7) |
| Via ear in inspiration + expiration | 10 (5.1) | 42 (28.2) | 52 (15.1) |
| Retraction | |||
| No | 13 (6.7) | 0 (0) | 13 (3.8) |
| Intercostal | 130 (66.7) | 34 (22.8) | 164 (47.7) |
| Tracheosternal | 52 (26.7) | 105 (70.5) | 157 (45.6) |
| Nasal wing | 0 (0) | 10 (6.7) | 10 (2.9) |
| General status | |||
| Well | 25 (12.8) | 0 (0) | 25 (7.3) |
| Anxious | 98 (50.3) | 15 (10.1) | 113 (32.8) |
| Anxious, decreased nutrition | 72 (36.9) | 83 (55.7) | 155 (45.1) |
| Lack of nutrition, altered consciousness | 0 (0) | 51 (34.2) | 51 (14.8) |
| Apnea | 0 (0) | 6 (4.0) | 6 (1.7) |
Examination of Physical Examination Findings of the Patients a
| Wang Score | Mild Mean (Min - Max) | Moderate Mean (Min - Max) | Severe Mean (Min - Max) | P-Value |
|---|---|---|---|---|
| HALP Score | 76.14 (26.31 - 138.98) | 53.34 (18.6 - 118.1) | 33.26 (10.51 - 138.98) | 0.00 |
| m-HALP Score | 14834315.37 (2778750 - 3.77E + 7) | 8780333.97 (1461600 - 3.42E + 7) | 6546097.83 (1475520 - 2.80E + 7) | 0.00 |
| NLR | 1.11 (0.29 - 5.5) | 1.57 (0.25 - 8.46) | 2.96 (0.38 - 9.75) | 0.00 |
| PLR | 69.80 (37.2 - 153.5) | 95.65 (32.6 - 245.14) | 174.43 (52.66 - 405.83) | 0.00 |
Examination of the Correlation Between Wang Score Levels and HALP, Modified-HALP, Neutrophil-to-Lymphocyte Ratio, and Platelet-to-Lymphocyte Ratio
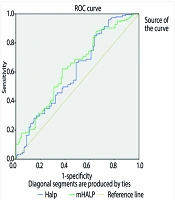
Receiver operating characteristic curve analysis was conducted to examine the diagnostic performance of HALP and m-HALP in predicting ICU hospitalization. It was observed that both scores were effective for predicting ICU hospitalization among hospitalized patients [area under the curve (AUC): 0.605 [95% CI 0.544 - 0.667] - AUC: 0.631 [95% CI 0.571 - 0.690]. This is shown in Figure 1 and Table 4.
| Accuracy Index | HALP Score | m-HALP Score |
|---|---|---|
| AUC | 0.605 | 0.631 |
| P-value | 0.001 | 0.000 |
| Cut-off value | 50.54 | 7645023 |
| Youden J Index | 0.05 | 0.25 |
| Sensitivity (95% CI) | 0.544 | 0.571 |
| Specificity (95% CI) | 0.667 | 0.690 |
Results of Receiver Operating Characteristic Curve Analysis on HALP Score and Modified-HALP Score in Terms of ICU Hospitalization
The cut-off values for the HALP and m-HALP scores were determined using the Youden index. The high value of the m-HALP score is due to the method used to calculate this score. The m-HALP score naturally has a higher value because it is obtained by multiplying the components of the HALP score.
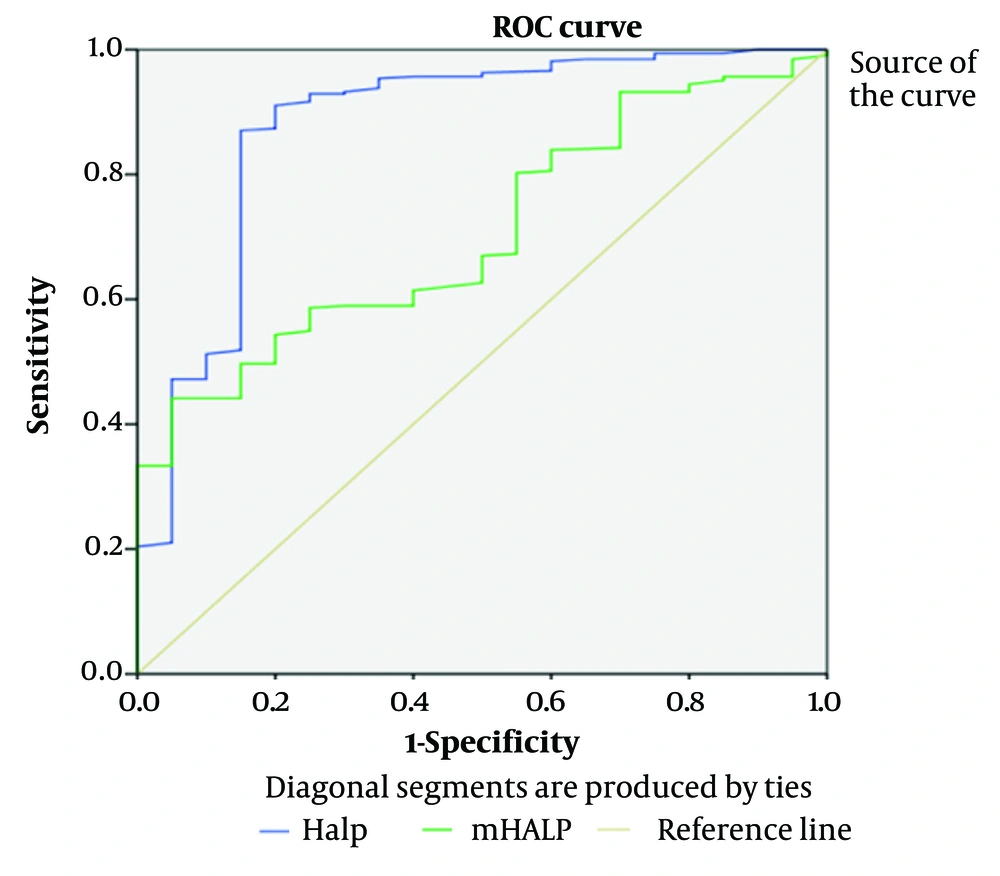
The ability of both scoring systems to predict the need for endotracheal intubation was examined. HALP and m-HALP scores (AUC: 0.879 and 0.707, respectively) were successful in predicting the need for endotracheal intubation. This is shown in Figure 2 and Table 5.
| Accuracy Index | HALP Score | m-HALP Score |
|---|---|---|
| AUC | 0.879 | 0.707 |
| P-value | 0.000 | 0.002 |
| Cut-off value | 36.41 | 6132825.6 |
| Youden J Index | 0.7 | 0.19 |
| Sensitivity (95% CI) | 0.785 | 0.612 |
| Specificity (95% CI) | 0.973 | 0.802 |
Results of Receiver Operating Characteristic Curve Analysis of HALP and Modified-HALP Scores in Terms of Predicting the Need for Endotracheal Intubation
Potential confounding factors (age, gender, and nutritional status) were controlled for by multivariate logistic regression analysis. This analysis confirmed the independent predictive value of the HALP and m-HALP scores in predicting the need for intensive care (OR for HALP: 1.85, 95% CI: 1.32 - 2.59, P < 0.001; OR for m-HALP: 1.92, 95% CI: 1.38 - 2.67, P < 0.001). In subgroup analyses, the performance of HALP and m-HALP scores was evaluated according to age groups (0 - 6 months, 6 - 12 months, 12 - 24 months) and nutritional status. The predictive value of the scores was similar across all subgroups.
To assess the potential biases in our study, the characteristics of the excluded patients were analyzed and compared with those of the included patients. No significant differences were found in this comparison, suggesting a low risk of selection bias.
5. Discussion
Predicting the prognosis of patients with acute bronchiolitis in the pediatric population is highly valuable for distinguishing critically ill patients and determining the need for hospitalization or intensive care. In the current study, the prognostic value of the recently established m-HALP score and the HALP score, which are used as prognostic markers in various diseases, was evaluated in patients with acute bronchiolitis.
According to the ROC analysis results, the diagnostic performance of HALP and m-HALP scores in predicting the need for intensive care was moderate (AUC for HALP: 0.605, 95% CI: 0.544 - 0.667; AUC for m-HALP: 0.631, 95% CI: 0.571 - 0.690). These values suggest that the scores may be useful in predicting bronchiolitis severity, but they have limitations when used alone.
The optimal cut-off values were 50.54 for HALP and 7 645 023 for m-HALP. The seemingly high value of the m-HALP score is due to the method of calculating this score. m-HALP naturally has a higher value because it is obtained by multiplying the components of the HALP score. This high value may make m-HALP difficult to interpret in clinical practice and highlights the need for standardization.
In this study, HALP and m-HALP scores were found to be effective parameters in predicting the need for ICU hospitalization, the need for intubation, and the identification of patients with a critical prognosis in pediatric patients with bronchiolitis. However, HALP and m-HALP scores were found to be similarly effective. Additionally, NLR and PLR were also shown to be effective parameters in discriminating critically ill patients.
The mechanistic association of HALP and m-HALP scores with bronchiolitis severity may be explained by the role of their components in disease pathophysiology (16). For example, a low albumin level may reflect an inflammatory response and increased vascular permeability, while a low hemoglobin level may indicate impaired tissue oxygenation. Lymphopenia may reflect suppression of the immune response to viral infection, and changes in platelet count may indicate an inflammatory response. The combination of these factors can help predict the severity and potential complications of bronchiolitis.
HALP and m-HALP scores are recently described valuable indices of systemic inflammation and nutritional status, used to assess the overall physiological status of patients based on albumin, hemoglobin, platelet, and lymphocyte values. Higher HALP and m-HALP scores have been associated with longer survival and better prognosis in previous studies (8, 10, 12, 17).
When comparing the findings of the present study with those reported in the literature, most results are similar. Notably, many previous studies focused on cancer patients, while the application of these scores in acutely infectious conditions like bronchiolitis is more limited (8, 10, 12, 17). For example, in a study by Kocaoglu and Alatli (8), the m-HALP score was more optimized for performance in patients suffering from acute heart failure, whereas in the current study, HALP and m-HALP scores showed similar effectiveness. This difference might be related to the distinct pathophysiological mechanisms of the diseases.
Chen et al. were the first to use this score to evaluate prognosis in gastric cancer patients (11). Since then, it has been employed to predict mortality in various diseases, particularly cancers. Xu et al. demonstrated that the HALP score could be an important parameter for postoperative survival and recurrence in patients with pancreatic cancer (17). Feng et al. showed that this score can serve as an independent prognostic method in esophageal cancer (18). Tian et al. found a correlation between the HALP score and mortality in their study of stroke patients (12). In a study by Cay and Duran it was shown that obese patients who underwent sleeve gastrectomy and had higher HALP scores lost more weight, and their laboratory values improved significantly (13). Kocaoglu and Alatli developed the m-HALP score and suggested that it provided better results than the classical HALP score in predicting 3-month mortality in patients with acute heart failure (8).
The first parameter that constitutes both scores is the hemoglobin level. Hemoglobin plays a key role in oxygen transport to tissues. Therefore, low hemoglobin (Hb) in patients with bronchiolitis presents a clinical risk. Another study involving 220 pediatric patients demonstrated that anemia worsens clinical outcomes in individuals with bronchiolitis. In a prospective study conducted by Hussain et al., involving children aged 1 month to 5 years, anemic patients were 4.6 times more likely to develop lower respiratory tract infections (19). The present study found no significant difference between ward patients and ICU patients in terms of Hb level. However, it was observed that the mean Hb level was lower in ICU patients.
The second laboratory value in the scores is albumin. Albumin, which functions as the primary protein in the intravascular space, is also a negative acute-phase reactant. Mansbach et al. showed that low albumin levels were associated with an increased risk of apnea in 1,016 infants with bronchiolitis (20). In this study, albumin levels were lower in patients with bronchiolitis who were hospitalized in the ICU. This contributed to the lower HALP and m-HALP scores observed in ICU patients.
Lymphocytes are produced from stem cells in the bone marrow, and their primary role is to combat pathogens such as bacteria, fungi, parasites, and viruses. Studies have shown that lymphopenia is associated with a worse prognosis in patients with bronchiolitis (21, 22). In this study, lymphocyte counts were significantly lower in the critically ill patient group, which is consistent with the literature. Lymphocytes constitute the third value in both scores.
Platelets play important roles in inflammation, hemostasis, angiogenesis, and tissue repair and regeneration. They also release mediators such as chemokines, cytokines, and coagulation factors. There is controversy regarding whether thrombocytosis or thrombocytopenia poses a greater risk in bronchiolitis. Some studies have suggested that thrombocytosis leads to a poor prognosis, while others have argued that thrombocytopenia is more relevant for critically ill patients with bronchiolitis (23, 24). Additionally, Sun et al. demonstrated that dynamic changes in platelet levels in patients with bronchiolitis may provide insights into patient prognosis (25). In this study, patients requiring intensive care had lower mean platelet levels; however, no statistically significant difference was observed.
The incorporation of HALP and m-HALP scores into real-life clinical scenarios can be beneficial for risk stratification and management of bronchiolitis patients. For example, these scores may aid in identifying patients at risk of deterioration who are likely to require mechanical ventilation and, therefore, are candidates for more aggressive treatment (25).
However, the intermediate sensitivity and specificity of the scores (0.54 and 0.67 for HALP, 0.57 and 0.69 for m-HALP) may present some limitations in clinical application. Therefore, it is recommended to assess these scores in combination with other clinical and laboratory parameters.
Our study has several limitations. First, there is a risk of selection bias due to the retrospective design. Second, being a single-center study may limit the generalizability of the results. Third, long-term follow-up data for patients were not available. Finally, not all potential confounding factors could be controlled. Recommendations for future studies include: (1) Confirming the efficacy of these scores in multicenter, prospective studies; (2) evaluating long-term outcomes; (3) investigating the combination of HALP and m-HALP scores with other prognostic markers; and (4) examining the performance of the scores in different bronchiolitis subtypes.
5.1. Conclusions
HALP and m-HALP scores are significant parameters that can be used to predict the need for intensive care, the need for endotracheal intubation, and identify critically ill patients in pediatric patients with acute bronchiolitis. These scores can provide valuable information for clinicians regarding patient follow-up and prognosis prediction. However, due to their moderate diagnostic performance, it is recommended that these scores be used in combination with other clinical and laboratory parameters. Future studies will help more clearly define the role of these scores in clinical practice.