1. Background
Chronic kidney disease (CKD) in pediatric patients is defined as kidney damage or a reduction in the glomerular filtration rate (GFR) persisting for a minimum of three months (1). Chronic kidney disease in children is associated with significant morbidity and mortality. Early identification of CKD progression is critical, as it enables timely therapeutic interventions, patient and family counseling, and the potential discovery of novel treatment strategies. However, predicting CKD progression in children remains challenging due to the heterogeneous nature of the disease and its variable course.
Currently, serum creatinine and proteinuria are the primary biomarkers used to monitor CKD progression (2). However, these markers typically show significant changes only in the advanced stages of the disease, reflecting substantial kidney damage, inflammation, and fibrosis (3). In the chronic kidney disease in children (CKiD) study, traditional markers such as proteinuria and serum creatinine accounted for only 32% of the variability in GFR decline (2), underscoring the need for additional biomarkers that can more accurately predict disease progression.
Recent studies have demonstrated that novel biomarkers identified in plasma and urine reflect diverse biological pathways, including tubular injury, tubular dysfunction, inflammation, and tubular health, and may serve as valuable indicators for CKD progression in pediatric patients (2, 4). However, there are limited studies evaluating the relationship between apoptosis, CKD progression, and inflammation in children. A substantial gap in longitudinal data regarding CKD progression in children remains, posing challenges to effective disease management.
Several molecular and cellular mechanisms contribute to renal fibrosis—the principal driver of progressive renal function decline in CKD—including oxidative stress, inflammation, endothelial cell injury, and apoptosis. Chronic inflammation in patients with CKD promotes the overexpression of both Fas and FasL, leading to enhanced Fas-FasL binding (5). Fas, a death receptor, and its ligand, FasL, belong to the TNF superfamily, which plays a critical role in regulating both physiological and pathological immune responses. The activation of Fas by FasL serves as a key initiator of the extrinsic apoptotic pathway (6, 7).
Fas and FasL exist in two primary forms: Membrane-bound (mFas and mFasL) and soluble [soluble Fas (sFas) and soluble Fas ligand (sFasL)]. While the pro-apoptotic role of the membrane-bound forms is well established, the functionality of their soluble counterparts remains contentious (8). For sFas, several isoforms have been identified, which may exert either anti-apoptotic or pro-apoptotic effects depending on the context (9). In contrast, evidence surrounding sFasL more consistently supports its anti-apoptotic role, with only limited pro-apoptotic activity observed (10).
During inflammatory responses, E-selectin, a member of the LEC-CAM family of adhesion molecules, facilitates leukocyte adhesion to the vessel wall and their migration to sites of inflammation (11). Fas, FasL, and E-selectin may play critical roles in various pathological processes, particularly apoptosis and endothelial dysfunction, which are pivotal to CKD progression.
This study is part of the PROGRESS project, which explored the longitudinal levels of serum and urinary Heat Shock Proteins (HSPs) in children with CKD, examining their relationship with clinical parameters of the disease (12). Heat Shock Proteins, acting as molecular chaperones, are synthesized in response to stressors such as infections to preserve cellular protein structure (13).
2. Objectives
The aims of this study were to evaluate the longitudinal changes in serum and urinary levels of sFas, sFasL, and sE-selectin in children with CKD and to determine their utility as novel biomarkers for CKD progression. Additionally, the interactions between these biomarkers and HSPs, previously studied in the PROGRESS project, were evaluated.
3. Methods
3.1. Study Design and Participants
A total of 173 children were enrolled in this prospective, multicenter study. The CKD group consisted of 117 patients (37 girls, 80 boys; median age 12.5 years, interquartile range 8.1 - 15.2 years) with CKD stages 2 - 5, who were under follow-up at participating pediatric nephrology centers. The CKD was defined and classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group criteria (1).
The etiologies of CKD were as follows: Congenital anomalies of the kidney and urinary tract (CAKUT) (n = 64), inborn errors of metabolism (n = 13), neurogenic bladder (n = 12), cystic kidney disease (n = 12), and glomerular disease (n = 4). At baseline, 30% of CKD patients had hypertension and were receiving antihypertensive treatment. The use of antihypertensive agents, other supportive therapies, and immunosuppressive medications among CKD patients is summarized in Appendix 1 in Supplementary File.
Patients were excluded from the study if they were older than 18 years, had stage 1 CKD, were undergoing dialysis, had received a renal transplant, or did not consent to participate in the study.
The control cohort consisted of 56 healthy children (27 girls, 29 boys; median age 10.9 years, interquartile range 9.1 - 14.5 years) under 18 years old, who applied to the social pediatrics outpatient clinic. They had no history of acute or chronic illness.
Median age did not differ between the CKD and control groups (P = 0.892). A higher proportion of boys was observed in the CKD group compared to the control group (P = 0.034).
Upon enrollment, both patients and healthy children underwent systemic physical examinations. The estimated glomerular filtration rate (eGFR) was determined using the Schwartz formula (14). The sample collection times for this study coincided with the patients' routine visits. In other words, all samples were collected on the same day that other biochemical parameters, such as hemoglobin, ferritin, serum calcium, phosphorus, and parathormone, were obtained, ensuring consistency in the data gathered. The results of these parameters were retrieved from the medical records.
Samples were collected at the initial assessment, the 12th month, and the 24th month for the CKD group, while samples were taken only once for the control group. Neither the patients nor the healthy children had any infections or underwent surgeries within 90 days prior to sampling.
CKD progression was defined as the need for renal replacement therapy and/or a 25% decline in eGFR from the initial assessment at the 24-month follow-up.
3.2. Measurement of Serum and Urinary Biomarkers
Serum and urine concentrations of sFas, sFasL, and sE-selectin were measured using the Luminex Human Magnetic Assay Kit (Cat. LXSAHM-3, R&D Systems) and the MAGPIX Multiplexing System [Millipore Sigma], following the manufacturer's protocol. Data were analyzed using Milliplex Analyst 5.1 data analysis software [Millipore Sigma]. The detection sensitivity for sFas, sFasL, and sE-selectin was 3.2 pg/mL, 1.2 pg/mL, and 18.8 pg/mL, respectively. Each sample was assessed in duplicate according to the manufacturer’s guidelines. The intra-assay coefficients of variation (CVs) for sFas, sFasL, and sE-selectin were < 20%, and the inter-assay CVs were < 25%. sFas, sFasL, and sE-selectin levels were expressed in pg/mL. All urine biomarkers were indexed to urine creatinine to adjust for urine concentration. The urinary ratios of sFas/Cr, sFasL/Cr, and sE-selectin/Cr were expressed in pg/mg. Serum and urine creatinine levels, as well as urine protein levels, were measured using an Architect c16000 analyzer (Abbott Laboratories, Abbott Park, IL, USA), with results reported in mg/dL. The urinary protein-to-creatinine ratio was expressed in mg/mg.
3.3. Power Calculation and Statistical Analysis
Based on the descriptive values of the primary variable in the study, a power analysis for repeated measurements (for 3-section audits) was performed. The required number of subjects in each group was calculated to be at least 60, assuming a Type 1 error probability of 0.05, a test power of 95%, and an effect size of 0.40. To ensure sufficient statistical power in subgroup analyses, the patient group was deemed to require 120 subjects.
Descriptive statistics are presented as median values and interquartile ranges. The chi-square test was employed to assess qualitative data.
Since none of the variables were normally distributed, as confirmed by the Kolmogorov–Smirnov test, differences between all groups and pairwise comparisons were evaluated using nonparametric tests (Kruskal-Wallis, Mann-Whitney U). The Friedman test was used to evaluate changes over time. The Wilcoxon test, with Bonferroni correction for multiple comparisons, was used for pairwise differences. Spearman’s correlation coefficient was applied to assess correlations between variables in the CKD patients. Univariate and subsequently multivariate logistic regression analyses were conducted using biomarkers to predict disease progression in children with CKD. Statistical analysis was performed using IBM SPSS Statistics version 22.0 for Windows (IBM Corp., Armonk, NY, USA). A P-value < 0.05 was considered significant.
4. Results
4.1. Comparison of sFas, sFasL, and sE-selectin Levels Between the Chronic Kidney Disease and Control Groups
At baseline, 12th month, and 24th month, the CKD group exhibited higher serum levels of sFas, sFasL, and sE-selectin compared to the control group, as well as higher urinary sFas/Cr, sFasL/Cr, and sE-selectin/Cr ratios (Table 1).
| Variables | Control Group | CKD Group | P-Value b | ||
|---|---|---|---|---|---|
| Baseline | 12th Month | 24th Month | |||
| Serum sFas (pg/mL) | 3951 (2818 - 6283) | 8337 (6696 - 11333) | 8262 (6357 - 10512) | 7773 (5904 - 10748) | 0.103 |
| P-value c | < 0.0001 | < 0.0001 | < 0.0001 | ||
| Urinary sFas/Cr (pg/mg) | 609 (354 - 1182) | 3369 (1876 - 9731) | 2750 (1474 - 6668) | 3318 (1353 - 6854) | 0.068 |
| P-value c | < 0.0001 | < 0.0001 | < 0.0001 | ||
| Serum sFasL (pg/mL) | 33 (23 - 56) | 68 (56 - 93) | 43 (31 - 70) | 51 (38 - 81) | < 0.0001 |
| P-value c | < 0.0001 | 0.008 | < 0.0001 | ||
| Urinary sFasL/Cr (pg/mg) | 7 (5 - 15) | 19 (9 - 38) | 11 (7 - 22) | 14 (8 - 22) | < 0.0001 |
| P-value c | < 0.0001 | 0.007 | 0.002 | ||
| Serum sE-selectin (pg/mL) | 19801 (12191 - 25565) | 26460 (22087 - 37315) | 23768 (18523 - 34141) | 25373 (17948 - 34547) | 0.003 |
| P-value c | < 0.0001 | 0.004 | 0.002 | ||
| Urinary sE-selectin/Cr (pg/mg) | 79 (35 - 165) | 581 (286 - 1272) | 366 (208 - 721) | 324 (151 - 672) | < 0.0001 |
| P-value c | < 0.0001 | < 0.0001 | < 0.0001 | ||
Abbreviations: CKD, chronic kidney disease; Cr, creatinine; sFas, soluble Fas; sFasL, soluble Fas Ligand; sE-selectin, soluble E-selectin.
a Values are expressed as median (IQR).
b Wilcoxon test: Comparing the results of CKD baseline with the results of CKD 24th month.
c Mann-Whitney U test: Comparing the CKD group with the control group.
4.2. Course of sFas, sFasL, and sE-selectin Levels Over Time in the Chronic Kidney Disease Group
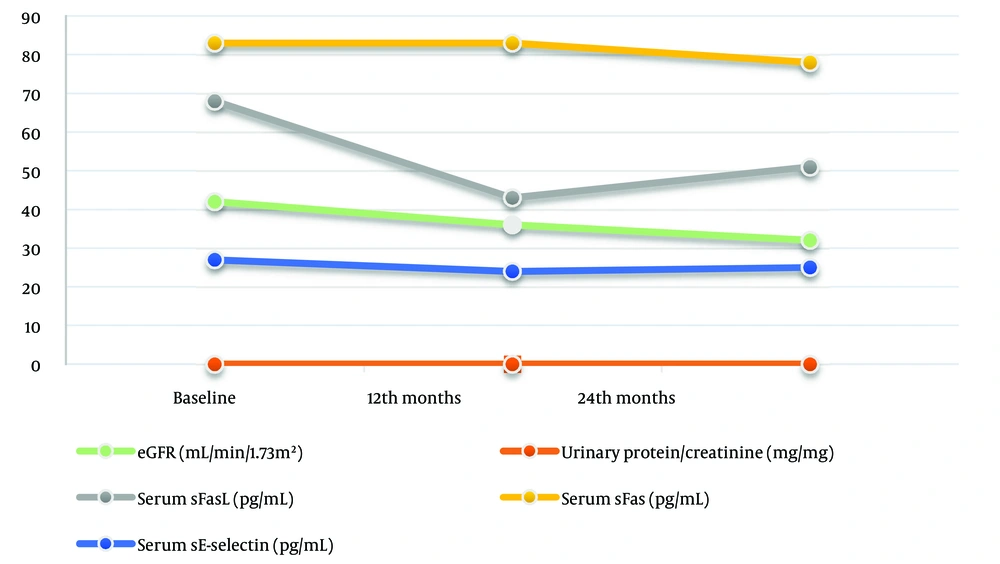
Although serum sFas levels gradually decreased over the two-year period, these differences did not reach statistical significance (P > 0.05) (Table 1 and Figure 1). The urinary sFas/Cr ratio did not show a significant difference when comparing baseline levels to the 24th month (P > 0.05); however, a significant decrease was observed from baseline to the 12th month (P = 0.004) (Table 1).
The levels of serum sFasL and urinary sFasL/Cr ratio decreased significantly at both the 12th and 24th months compared to baseline measurements (P < 0.0001 for both) (Figure 1). However, both levels increased at the 24th month compared to the 12th month, although this difference was not statistically significant (P > 0.05) (Table 1).
Serum sE-selectin levels decreased significantly at the 12th and 24th months compared to baseline (P < 0.0001 and P = 0.003, respectively) (Figure 1). There was no significant difference when comparing the results of the 12th and 24th months (P > 0.05) (Table 1). The urinary sE-selectin/Cr ratio steadily decreased at both the 12th and 24th months compared to baseline (P < 0.0001 and P = 0.001, respectively). The decrease continued at the 24th month compared to the 12th month, but the difference was not significant (P > 0.05).
4.3. Comparison of sFas, sFasL, and sE-selectin Levels Across Different Chronic Kidney Disease Stages at Initial Assessment
Baseline urinary sFas/Cr, sFasL/Cr, and sE-selectin/Cr ratios were highest in CKD stages 4 - 5 compared to CKD stages 2, 3a, and 3b (P < 0.0001, P < 0.0001, and P = 0.007, respectively). However, there were no differences in baseline serum sFas, sFasL, and sE-selectin levels among patients at different CKD stages (P > 0.05) (Table 2).
| Variables | Baseline CKD Group | P-Value b | |||
|---|---|---|---|---|---|
| CKD Stage 2 (n = 17) | CKD Stage 3a (n = 35) | CKD Stage 3b (n = 33) | CKD Stage 4 + 5 (n = 32) | ||
| Serum sFas (pg/mL) | 7194 (6291 - 9740) | 7926 (6457 - 10040) | 10153 (7124 - 12664) | 8767 (6193 - 12368) | 0.085 |
| Serum sFasL (pg/mL) | 66 (56 - 90) | 63 (52 - 80) | 66 (61 - 89) | 84 (60 - 133) | 0.163 |
| Serum sE-selectin (pg/mL) | 26404 (14480 - 30721) | 27269 (19352 - 39059) | 25911 (23527 - 42962) | 27152 (21995 - 33005) | 0.374 |
| Urinary sFas/Cr (pg/mg) | 1862 (704 - 2640) | 2641 (1516 - 4868) | 3879 (1951 - 12034) | 9238 (4957-14973) | < 0.0001 |
| Urinary sFasL/Cr (pg/mg) | 10 (4 - 14) | 18 (7 - 37) | 19 (12 - 33) | 28 (20 - 59) | < 0.0001 |
| Urinary sE-selectin/Cr (pg/mg) | 236 (173 - 582) | 431 (282 - 1267) | 623 (440 - 1305) | 860 (438 - 1451) | 0.007 |
Abbreviations: CKD, chronic kidney disease; Cr, creatinine; sFas, soluble Fas; sFasL, soluble Fas ligand; sE-selectin, soluble E-selectin.
a Values are expressed as median (IQR).
b Kruskal-Wallis test for comparing among the CKD stages.
4.4. Correlations Between Baseline Levels of sFas, sFasL, and sE-selectin and Age, Gender, and eGFR
Age showed a positive correlation with serum sFas levels (r = 0.293, P = 0.001) and a negative correlation with serum sFasL levels (r = -0.466, P < 0.0001), urinary sFas/Cr ratio (r = -0.199, P = 0.032), urinary sFasL/Cr ratio (r = -0.275, P = 0.003), and urinary sE-selectin/Cr ratio (r = -0.293, P = 0.001). There was no difference between girls and boys in terms of other parameters except serum sE-selectin (P > 0.05), with the median serum sE-selectin values being higher in boys than in girls (P = 0.009). The eGFR was negatively correlated with serum sFasL levels (r = -0.202, P = 0.029), and urinary sFas/Cr ratio (r = -0.500, P < 0.0001), sFasL/Cr ratio (r = -0.393, P < 0.0001), and sE-selectin/Cr ratio (r = -0.305, P = 0.001).
4.5. Correlations Between Baseline Levels of sFas, sFasL, and sE-selectin and Biochemical Parameters
Serum Hb levels were negatively correlated with urinary sFas/Cr ratio (r = -0.238, P = 0.010), urinary sFasL/Cr ratio (r = -0.245, P = 0.008), and urinary sE-selectin/Cr ratio (r = -0.199, P = 0.033). Serum ferritin levels were negatively correlated with urinary sFasL/Cr ratio (r = -0.206, P = 0.047). There was no correlation between serum levels of sFas, sFasL, and sE-selectin and serum levels of calcium, phosphorus, parathyroid hormone (PTH), and hemoglobin (Hb) (P > 0.05), except for a positive correlation between serum sFas and PTH levels. The urinary protein/Cr ratio correlated positively with urinary sFas/Cr ratio (r = 0.703, P < 0.0001), sFasL/Cr ratio (r = 0.663, P < 0.0001), and sE-selectin/Cr ratio (r = 0.716, P = 0.001) (Appendix 2 in Supplementary File).
4.6. Correlations Among Baseline Levels of sFas, sFasL, and sE-selectin
Serum sFas levels showed a negative correlation with serum sFasL levels (r = -0.239, P = 0.009) and a positive correlation with serum sE-selectin levels (r = 0.443, P < 0.0001). Serum sFasL levels were positively correlated with urinary sFasL/Cr ratio (r = 0.250, P = 0.007). Serum sE-selectin levels were positively correlated with urinary sE-selectin/Cr ratio (r = 0.319, P < 0.0001). Urinary sFas/Cr ratio was positively correlated with both urinary sFasL/Cr ratio (r = 0.805, P < 0.0001) and sE-selectin/Cr ratio (r = 0.726, P < 0.0001). Urinary sE-selectin/Cr ratio was positively correlated with urinary sFasL/Cr ratio (r = 0.845, P < 0.0001) (Appendix 3 in Supplementary File).
4.7. Correlations Between Baseline Levels of sFas, sFasL, and sE-selectin and HSPs
Serum sFasL was positively correlated with serum anti-HSP70 and negatively correlated with serum anti-HSP60 (r = 0.247, P = 0.007; r = -0.217, P = 0.019, respectively). Serum sFas and sE-selectin were not correlated with any serum HSPs (P > 0.05) (Appendix 4 in Supplementary File). Urinary sFas/Cr, sFasL/Cr, and sE-selectin/Cr ratios were positively correlated with all urinary HSP/Cr ratios (Table 3).
| Variables | Urinary sFas/Cr | Urinary sFasL/Cr | Urinary sE-selectin/Cr |
|---|---|---|---|
| Urinary HSP27/Cr | |||
| r | 0.673 | 0.768 | 0.654 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
| Urinary HSP40/Cr | |||
| r | 0.696 | 0.823 | 0.678 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
| Urinary HSP47/Cr | |||
| r | 0.610 | 0.643 | 0.547 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
| Urinary HSP60/Cr | |||
| r | 0.692 | 0.802 | 0.664 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
| Urinary HSP70/Cr | |||
| r | 0.701 | 0.823 | 0.673 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
| Urinary HSP72/Cr | |||
| r | 0.554 | 0.731 | 0.634 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
| Urinary HSP90/Cr | |||
| r | 0.392 | 0.483 | 0.339 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 |
Abbreviations: Cr, creatinine; HSPs: heat shock proteins; sFas: soluble Fas; sFasL: soluble Fas Ligand; sE-selectin: soluble E-selectin; r-Spearman’s correlation coefficient.
4.8. Comparison of Groups with and without Rapid Chronic Kidney Disease Progression
We assessed whether baseline levels of sFas, sFasL, and sE-selectin could predict rapid CKD progression. Baseline serum sFasL median levels were lower (P = 0.038), whereas baseline serum sFas median levels were higher (P = 0.045) in patients with rapid CKD progression compared to those without rapid progression. However, serum sE-selectin and urinary ratios of sFas, sFasL, and sE-selectin did not differ between the two groups (P > 0.05, Table 4). Age, gender, serum sFas, sFasL, sE-selectin, parathyroid hormone, baseline eGFR, urinary sFas/Cr, sFasL/Cr, sE-selectin/Cr, and protein/Cr ratio were evaluated using univariate logistic regression analysis to predict CKD progression. Variables with potential significance—serum sFas, sFasL, and parathyroid hormone—were further assessed through multivariate regression analysis. The odds ratios for these biomarkers were close to 1, indicating that baseline biomarkers did not reliably predict rapid CKD progression.
| Variables | With CKD Progression (n = 61) | Without CKD Progression (n = 56) | P-Value |
|---|---|---|---|
| Age (y) | 13.2 (9.8 - 15.2) | 10.5 (7.2 - 15.2) | 0.084 |
| Female/male ratio | 23/38 | 14/42 | 0.140 |
| sFas-ligand (pg/mL) | 64 (54 - 86) | 74 (58 - 109) | 0.038 |
| uFas-ligand/kreatinin(pg/mg) | 21 (11 - 34) | 18 (8 - 42) | 0.402 |
| sE-selectin (pg/mL) | 26427 (22087 -36928) | 26788 (20734 - 38572) | 0.956 |
| uE-selektin/kreatinin (pg/mg) | 775 (356 - 1277) | 462 (219 - 1142) | 0.107 |
| sFas (pg/mL) | 8843 (7124 - 11685) | 7501 (5632 - 10645) | 0.045 |
| uFas/kreatinin(pg/mg) | 4055 (1951 - 11115) | 2679 (1697 - 8601) | 0.399 |
| uProtein/Cr mg/mg | 1.1 (0.5 - 3.0) | 0.7 (0.2 - 1.5) | 0.013 |
| eGFR baseline mL/min/1.73 m2 | 36.2 (26.3 - 49.0) | 50.0 (33.0 - 59.8) | 0.005 |
Abbreviations: CKD, chronic kidney disease; sFas, soluble Fas; sFas-ligand, soluble Fas-ligand; sE-selectin, soluble E-selectin; eGFR, estimated glomerular filtration rate.
a Values are expressed as median (IQR).
4.9. Comparison of Patients with and without CAKUT in the Chronic Kidney Disease Group According to Etiology
Chronic kidney disease patients were divided into two groups based on etiology: Congenital anomalies of the kidney and urinary tract and non-CAKUT. There were no differences between the two groups in terms of serum sFas, sFasL, and sE-selectin levels, or urinary sFas/Cr, sFasL/Cr, and sE-selectin/Cr ratios (P > 0.05).
4.10. Comparison of Patients with and without Hypertension in the Chronic Kidney Disease Group
No significant differences were observed in serum sFas, sFasL, and sE-selectin levels, as well as in urinary sFas/Cr, sFasL/Cr, and sE-selectin/Cr ratios, between hypertensive and non-hypertensive patients within the CKD group (P > 0.05).
5. Discussion
Our study is the first to investigate the course of sFas, sFasL, and sE-selectin over the study period in pediatric patients with CKD and their associations with CKD progression. Our results demonstrate that children with CKD exhibit significantly elevated levels of serum sFas and sFasL, along with increased urinary ratios of sFas/Cr and sFasL/Cr, compared to healthy controls.
Adult patients with CKD have been found to have higher plasma sFas levels compared to healthy individuals (15-17). Similarly, elevated serum concentrations of both sFas and sFasL have been reported in pre-dialysis children with CKD and in children on chronic dialysis compared to healthy controls (18-20). A distinguishing aspect of our study, which corroborates these findings, is that we also evaluated the levels of these biomarkers in urine. There are very few studies in the literature evaluating sFas and sFasL in urine in children with CKD.
Our study not only confirmed the elevated serum and urine levels of sFas and sFasL in the CKD cohort relative to controls, but also elucidated that the urinary sFas/creatinine (Cr) and sFasL/Cr ratios were significantly higher in patients with CKD stages 4 - 5 compared to those in earlier stages of the disease. These results suggest a potential association between the stage of CKD and increased urinary excretion of these soluble markers, which may reflect a correlation with the extent of renal injury and inflammation. However, it is noteworthy that serum levels of sFas and sFasL did not vary significantly across CKD stages, highlighting the complexity of systemic versus local responses in CKD pathophysiology.
Interestingly, serum levels of sFas were higher, while serum levels of sFasL were lower in the CKD progression group compared to those who did not experience CKD progression. This observation suggests a differential role of these biomarkers in the trajectory of CKD. The negative correlation observed between serum sFas and sFasL levels may reflect a regulatory balance, possibly influenced by compensatory mechanisms in response to inflammation or kidney injury. Moreover, the inverse correlations between eGFR and both serum sFasL levels and sFasL/Cr ratios further emphasize the potential role of these markers in reflecting renal function decline. Collectively, these findings imply that sFasL may exert an anti-apoptotic effect, while sFas may play a pro-apoptotic role in the context of CKD progression.
Another significant strength of our study was the longitudinal observation of these biomarkers over several years. Serum sFasL levels and urinary sFasL/Cr ratios decreased significantly over the two-year period, while serum sFas levels and urinary sFas/Cr ratios showed no significant change over the same period. The decrease in sFasL may indicate that anti-apoptotic defenses or compensatory mechanisms become impaired as CKD progresses. However, whether this reduction directly impacts apoptosis remains unclear, as soluble forms of Fas and FasL can have variable effects on cell survival.
Endothelial dysfunction is characterized by increased oxidative stress, chronic inflammation, leukocyte adhesion, hyperpermeability, and vascular stiffening, all of which significantly contribute to the pathogenesis of CKD (21). E-selectin, a circulating biomarker reflecting endothelial dysfunction, has been observed at elevated levels in adult patients with CKD; however, there is a lack of extensive research on this biomarker in pediatric patients with CKD (21-25). Musial and Zwolinska (19) reported that serum concentrations of sE-selectin in pre-dialysis pediatric patients were significantly higher than in the control group. Similarly, our research noted elevated serum levels of sE-selectin and urinary ratios of sE-selectin/Cr in children with CKD relative to controls. Notably, the baseline urinary sE-selectin/Cr ratio was highest in CKD stages 4 - 5 compared to CKD stages 2, 3a, and 3b, and a negative correlation was observed between the urinary sE-selectin/Cr ratio and eGFR. However, baseline serum sE-selectin levels did not differ between patients with rapid CKD progression and those without rapid progression. The positive correlation between serum sE-selectin and sFas further supports the notion of apoptosis being implicated in this pathological process, suggesting that E-selectin may play a role in the apoptotic mechanisms associated with CKD.
The regulation of apoptosis involves several complex cellular pathways. One class of apoptosis regulators, although not widely recognized, is the HSPs. HSPs serve as molecular chaperones in unstressed cells and facilitate cell survival under both acute and chronic stress conditions (26). While the capacity of HSPs to inhibit stress-induced apoptosis is widely accepted, there are few studies exploring their effects on apoptosis induced through the extrinsic pathway. HSP27 is involved in both extrinsic and intrinsic apoptotic pathways. It inhibits apoptosis extrinsically by preventing the interaction between Fas and its receptor, FasL, and intrinsically by inhibiting the release of cytochrome c (27). Mehlen et al. (28) demonstrated that the continuous expression of human HSP27 in murine L929 cells inhibits Fas/APO-1-mediated cell death. Similarly, Clemons et al. (29) found that HSP72 inhibits apoptosis induced by Fas signaling, though this effect is limited to type II cells where the intrinsic pathway is necessary for initiating apoptosis. However, there is currently no data on potential connections between HSPs and the sFas/sFasL system in the progression of CKD.
In our initial study, baseline serum levels of HSP40, HSP47, HSP60, HSP70, anti-HSP60, and anti-HSP70 were found to be elevated in the CKD group compared to the control group. Notably, urinary sFas/Cr, sFasL/Cr, and sE-selectin/Cr ratios showed strong positive correlations with all urinary HSP/Cr ratios. These results suggest that HSPs, along with sFas, sFasL, and sE-selectin, may play a role in CKD progression. HSPs are recognized for their vital role in protecting cells from stress and apoptosis. However, their interaction with the extrinsic apoptotic pathway, specifically involving Fas and FasL, may include additional factors and signaling pathways that our study did not capture. Further investigation is needed to clarify the specific mechanisms through which HSPs influence the extrinsic apoptotic pathway and to identify other potential mediators involved in this process in the context of CKD.
In conclusion, we observed significantly elevated serum and urinary levels of sFas, sFasL, and sE-selectin in children with CKD. Furthermore, serum sFasL and sE-selectin levels, as well as urinary sFasL/Cr and sE-selectin/Cr ratios, showed a tendency to decrease over time. These findings may suggest a diminishing protective role of sFasL and sE-selectin in CKD as time progresses. The finding that serum sFas levels were higher, while serum sFasL levels were lower, in patients with rapid CKD progression suggests differential roles for these markers in CKD progression, with sFas potentially promoting and sFasL possibly inhibiting apoptosis.
Moreover, our study is among the first to report significant correlations between urinary HSP levels and urinary sFas, sFasL, and sE-selectin, suggesting that HSPs may collaborate with these biomarkers in CKD progression, possibly through mechanisms involving apoptotic regulation. While our findings contribute valuable information to the limited literature on these biomarkers in pediatric CKD, the relatively small sample size and limited duration of follow-up are notable limitations. Larger-scale, long-term studies are essential to clarify the mechanistic pathways linking these biomarkers to CKD pathophysiology, particularly the interaction between HSPs and the extrinsic apoptotic pathway, and to establish their utility as reliable prognostic markers for CKD progression in children.