1. Background
Cholestatic liver diseases are a leading cause of morbidity and mortality and represent the primary indication for pediatric liver transplantation. Conditions such as biliary atresia, Alagille syndrome, progressive intrahepatic cholestasis, ductal plate abnormalities (e.g., Caroli syndrome and congenital hepatic fibrosis), primary sclerosing cholangitis (PSC), bile acid synthesis disorders, and various metabolic conditions fall under this category (1).
The gut and liver maintain a bidirectional relationship mediated through the gut microbiota. The portal system facilitates the transport of blood from the gut, allowing intestinal blood contents to influence liver functions. Conversely, bile secretion from the liver provides feedback to the intestinal lumen. In addition to the direct injury caused by various etiological agents, modifications of the intestinal microbiota appear to play an essential role in the development and progression of liver damage. As a result, managing microbial communities is crucial for maintaining homeostasis within the gut-liver axis. Moreover, the liver can independently affect intestinal microbial communities as part of this bidirectional communication. Bacterial overgrowth, immunological dysfunction, changes in luminal factors, and increased intestinal permeability are all involved in the pathophysiology of liver cirrhosis complications such as infections, hepatic encephalopathy, spontaneous bacterial peritonitis, and renal failure (2-4).
In recent years, there has been a growing interest in the role of probiotics in modulating gut flora, with evidence suggesting their potential as a therapeutic option for various chronic liver conditions. This is most likely due to their capacity to improve intestinal barrier function and inhibit bacterial translocation (2, 5). According to current research, manipulation of probiotics within the gut microbiome may slow the progression of liver disease. Probiotics have also been demonstrated to reduce the severity of hepatic encephalopathy, lower hepatic venous pressure gradients, improve liver biochemistry, and reduce post-transplant infection rates (6-8). However, the exact mechanism by which gut microbial modulation alleviates cirrhosis has not yet been established (9).
Several studies have highlighted the beneficial effects of probiotics in nonalcoholic fatty liver disease (NAFLD) in children and in certain cholestatic liver conditions in adults. We hypothesized that a course of probiotic supplementation might improve or stabilize disease progression in children with cholestatic liver diseases. To test this hypothesis, we conducted a randomized, triple-blind, placebo-controlled trial to evaluate the effects of Lactobacillus sporogenesis supplementation in children with cholestatic liver diseases (10).
2. Methods
2.1. Study Population and Design
This triple-blind, parallel, placebo-controlled randomized clinical trial was conducted on children with cirrhosis registered in the Shiraz pediatrics liver cirrhosis cohort study (SPLCCS) from August 2020 to May 2021 at Motahari Clinic, Shiraz, Iran (IR.SUMS.REC.1398.142).
The SPLCCS enrolled children under 18 years of age with chronic liver disease who were referred to the cohort clinic by pediatric gastroenterologists from across Iran (11).
Children with a confirmed diagnosis of liver disease with a cholestatic etiology were selected by a pediatric gastroenterologist to participate in the study. Exclusion criteria included: Antibiotic use within four weeks prior to enrollment; gastrointestinal bleeding or spontaneous bacterial peritonitis within the past two months; active microbial infection; high-protein diet; use of immunosuppressive drugs or immunocompromised status due to underlying conditions; hypersensitivity to probiotics; renal failure (creatinine > 1.5 mg/dL adjusted for age and sex); and electrolyte imbalances (serum sodium < 130 or > 150 meq/L, serum potassium < 3.0 or > 5.5 meq/L).
2.2. Recruitment and Randomization of Participants
Based on the study objectives and prior research, a sample size of 28 participants per group was calculated to provide 80% power with a 5% significance level. The effect size was approximately 75% (mean difference = 20, SD1 = 31, and SD2 = 22), using a 1:1 ratio.
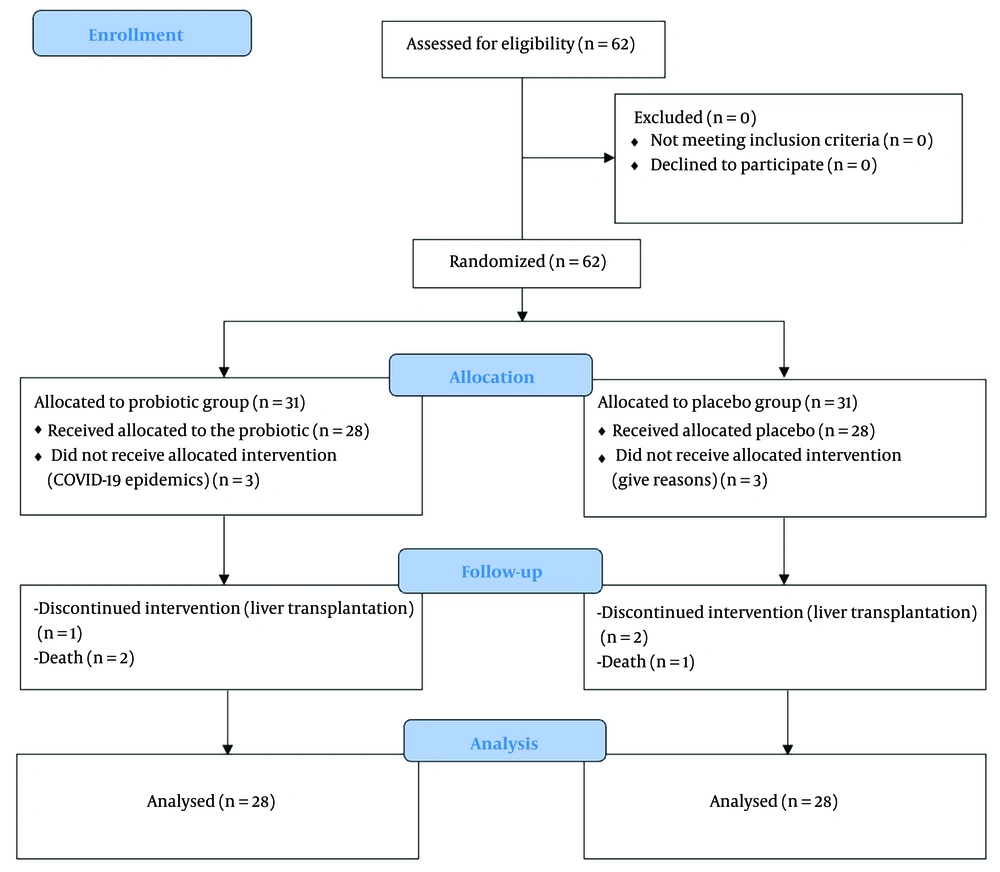
Eligible children were recruited into the study using a convenience sampling approach. A trained cohort staff member employed a permuted block randomization method with quadruple blocks. Participants were randomly assigned to either the probiotic or placebo group using this method. Children who consumed probiotics were labeled as group A, and those who received the placebo were classified as group B. Participants, investigators, and researchers who extracted and analyzed the data were blinded to the assigned groups until the codes were revealed after statistical analysis (Figure 1).
2.3. Intervention
All children were instructed to take 15 drops of the solution (probiotics or placebo), daily—five drops with each main meal—for four weeks. They continued their routine medications during the study. Parents or caregivers were advised not to administer any other probiotics to the children during the study period. The investigators monitored the patients through phone calls throughout the process to ensure proper usage, address any questions, and document any potential complaints. Parents reported no complications associated with probiotic use.
2.4. Data Gathering
Baseline data, including age, sex, underlying disease, comorbidities, and anthropometric measurements, were obtained from the Pediatric Liver Cirrhosis Registry (IR.SUMS.REC.1399.530). Laboratory data, including complete blood count (CBC), liver function tests (LFTs), and coagulation parameters, were assessed as part of routine follow-up during enrollment and again two weeks after the study period. The pediatric end-stage liver disease (PELD) score, used as an overall assessment of liver disease progression, was calculated using Medscape medical calculator at enrollment and six weeks later. Laboratory values less than 1.0 were rounded up to 1.0 for the purpose of the PELD score calculation.
2.5. Ethical Consideration
All procedures adhered to the principles outlined in the Declaration of Helsinki and were approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1399.277). The confidentiality of personal information and the voluntary nature of participation were fully assured. All participants were provided with detailed information about the study protocol. Written informed consent was obtained from their parents or legal representatives. The study protocol was registered in the Iranian Registry of Clinical Trials (IRCT20200628047940N1).
2.6. Probiotics Preparation
To prepare the probiotic oral syrup, the bacterium Bacillus coagulans (formerly known as L. sporogenesis) was cultivated. The initial inoculum, obtained from the Biotechnology Research Center in Shiraz, Iran, had been previously characterized and deposited in the National Center for Biotechnology Information (NCBI) database.
The sporulation medium was prepared following standardized protocols and contained 10 g soy peptone, 2 g potassium hydrogen phosphate, 20 g maltose, 10 g yeast extract, and 2 g glucose per liter. The culture was incubated at 38 ± 1°C for 35 - 37 hours with agitation at 200 rpm to achieve an 80 - 90% sporulation efficiency, verified via phase-contrast microscopy.
After incubation, the culture was centrifuged (10,000 × g, 15 minutes, 4°C), and the pellet was washed twice with sterile deionized water before being resuspended in a protective medium containing 10% (w/v) skim milk as a cryoprotectant. The spore suspension was rapidly frozen using liquid nitrogen or freeze-dried (-40°C for 2 hours, 0.1 mbar vacuum, -55°C condenser, 48 hours). The resulting freeze-dried spores were stored at 4°C in airtight sterile containers.
For the oral syrup formulation, the freeze-dried B. coagulans spores were incorporated into a pharmaceutical-grade medium-chain triglyceride (MCT) oil at a concentration of 5% (w/v), ensuring a final viable count of 1.5 × 108 colony forming unit (CFU)/mL. The mixture was homogenized at 5000 rpm for 10 minutes, with silicon dioxide (2% w/v) added as an anti-caking agent to improve stability and flow properties. The suspension was further mixed at 3000 rpm for 5 minutes before being aseptically filled into 60 mL amber glass bottles with calibrated droppers for accurate dosing.
2.6.1. Dosing Considerations
The dosage was standardized to deliver a consistent CFU count per drop, ensuring practical administration for pediatric use. While individual adjustments based on weight or age were considered, the formulation was designed to be suitable for the intended population based on existing literature and clinical recommendations.
The calculated dosage for administration is 1 billion CFU/day for adults and 100 million CFU/day for babies and infants. Considering that our product contains 2 billion CFU/mL, the required amounts for administration are as follows: (1) For adults, 10 droplets (0.05 mL per droplet) are needed to provide 1 billion CFU; (2) for babies and infants, approximately 1 - 2 droplets are sufficient to deliver 100 million CFU. This calculation ensures precise dosing based on CFU counts, aligning with standard practices in probiotic administration as supported by the literature and commercial formulations.
For the placebo, an identical formulation was prepared, containing only MCT oil (98% w/v) and silicon dioxide (2% w/v), without B. coagulans spores. The placebo was indistinguishable from the active formulation in taste, appearance, and packaging, ensuring effective blinding for clinical evaluation (12).
2.7. Statistical Analyses
Data were presented as mean ± standard deviation (SD) for continuous variables and as frequency (percentage) for categorical variables. Chi-square and t-tests were used to evaluate the statistical significance of differences between the groups. The difference-in-difference (Diff-in-Diff) method was applied to assess the effectiveness of the intervention. All analyses were performed using SPSS, version 22 (SPSS Inc., Chicago, IL, USA). P-value < 0.05 was considered statistically significant.
3. Results
A total of 62 children were initially enrolled in the study; three patients died during follow-up, and three underwent liver transplantation. Consequently, data from 56 patients were analyzed (group A: N = 28, group B: N = 28).
At baseline, there were no significant differences between the probiotic and placebo groups in terms of age, sex, anthropometric measurements, or underlying diseases (Table 1), indicating a well-balanced study population.
| Variables | Group A (N = 28) | Group B (N = 28) | P-Value |
|---|---|---|---|
| Age (y) | 1.91 ± 1.94 | 2.66 ± 2.48 | 0.212 |
| Male gender | 16 (57.1) | 9 (32.1) | 0.060 |
| Height (cm) | 74.43 ± 17.67 | 75.86 ± 15.59 | 0.750 |
| Weight (kg) | 8.74 ± 3.61 | 10.24 ± 4.25 | 0.162 |
| Head circumference (cm) | 43.24 ± 4.97 | 44.97 ± 5.79 | 0.236 |
| Underlying diseases | |||
| Biliary atresia | 18 (64.3) | 13 (46.4) | 0.179 |
| PFIC | 10 (35.7) | 15 (53.6) | 0.179 |
a Values are expressed as No. (%) or mean ± SD.
The PELD score significantly decreased in the probiotic group (group A) from 19.25 ± 9.20 to 15.27 ± 8.94 (P = 0.025), while no significant change was observed in the placebo group (14.64 ± 11.79 to 13.10 ± 11.02, P = 0.468). The mean change in PELD score was -3.98 ± 8.88 in the probiotic group and -1.54 ± 11.07 in the placebo group, with an effect size of 0.24 and a 95% confidence interval (CI) of -7.81 to 2.93 (P = 0.367).
For albumin levels, no significant change was noted in the probiotic group (3.58 ± 0.74 to 3.43 ± 1.02, P = 0.471). However, in the placebo group, albumin levels significantly decreased from 4.02 ± 0.69 to 3.74 ± 0.86 (P = 0.009). The mean change in albumin levels was -0.15 ± 1.10 in the probiotic group and -0.28 ± 0.53 in the placebo group, with an effect size of 0.15 and a 95% CI of -0.33 to 0.59 (P = 0.575).
International normalized ratio (INR) levels significantly increased in both groups, with a mean increase of 0.78 ± 1.99 in the probiotic group (P = 0.047) and 1.02 ± 1.85 in the placebo group (P = 0.007). The effect size for INR changes was 0.12, with a 95% CI of -1.27 to 0.78 (P = 0.638).
No statistically significant differences were found between the two groups in total bilirubin, white blood cell count, red blood cell count, hemoglobin levels, liver enzymes (AST, ALT, ALP), or other biochemical markers after the intervention (Table 2).
| Variables | Group A (N = 28) | Group B (N = 28) | 95% CI | Effect Size | P-Value |
|---|---|---|---|---|---|
| PELD score | |||||
| First | 19.25 ± 9.20 | 14.64 ± 11.79 | -1.05, 10.28 | - | 0.109 |
| Second | 15.27 ± 8.94 | 13.10 ± 11.02 | -3.20, 7.55 | - | 0.422 |
| Change | -3.98 ± 8.88 | -1.54 ± 11.07 | -7.81, 2.93 | 0.24 | 0.367 |
| P-value | 0.025 | 0.468 | - | - | - |
| White blood cells (109/L) | |||||
| First | 9.97 ± 4.50 | 8.63 ± 2.25 | -0.94, 9.22 | - | 0.165 |
| Second | 10.23 ± 4.69 | 8.82 ± 3.40 | -3.78, 8.81 | - | 0.203 |
| Change | 0.25 ± 5.32 | 0.18 ± 2.41 | -6.56, 3.24 | 0.01 | 0.950 |
| P-value | 0.808 | 0.690 | - | - | - |
| Red blood cells (1012/L) | |||||
| First | 4.14 ± 0.69 | 4.30 ± 0.78 | -0.56, 0.23 | - | 0.421 |
| Second | 3.90 ± 0.75 | 4.16 ± 0.63 | -0.62, 0.11 | - | 0.177 |
| Change | -0.24 ± 0.90 | -0.14 ± 0.62 | -0.50, 0.32 | 0.12 | 0.654 |
| P-value | 0.167 | 0.218 | - | - | - |
| Hemoglobin (g/dL) | |||||
| First | 10.67 ± 1.83 | 10.40 ± 1.45 | -0.71, 1.06 | - | 0.699 |
| Second | 10.31 ± 1.57 | 10.06 ± 1.58 | -0.60, 1.09 | - | 0.536 |
| Change | -0.36 ± 1.58 | -0.43 ± 1.34 | -0.71, 0.86 | 0.05 | 0.853 |
| P-value | 0.233 | 0.094 | - | - | - |
| Total bilirubin (mg/dL) | |||||
| First | 14.18 ± 10.18 | 10.04 ± 8.74 | -0.94, 9.22 | - | 0.109 |
| Second | 13.59 ± 11.81 | 11.07 ± 11.69 | -3.78, 8.81 | - | 0.426 |
| Change | -0.59 ± 9.50 | 1.02 ± 8.94 | -6.56, 3.32 | 0.17 | 0.514 |
| P-value | 0.745 | 0.548 | - | - | - |
| Albumin (g/dL) | |||||
| First | 3.58 ± 0.74 | 4.02 ± 0.69 | -0.82, -0.05 | - | 0.025 |
| Second | 3.43 ± 1.02 | 3.74 ± 0.86 | -0.82, 0.19 | - | 0.223 |
| Change | -0.15 ± 1.10 | -0.28 ± 0.53 | -0.33, 0.59 | 0.15 | 0.575 |
| P-value | 0.471 | 0.009 | - | - | - |
| INR | |||||
| First | 1.63 ± 0.56 | 1.50 ± 0.70 | -0.20. 0.47 | - | 0.434 |
| Second | 2.41 ± 1.99 | 2.52 ± 2.41 | -1.29, 1.07 | - | 0.853 |
| Change | 0.78 ± 1.99 | 1.02 ± 1.85 | -1.27, 0.78 | 0.12 | 0.638 |
| P-value | 0.047 | 0.007 | - | - | - |
| Aspartate transaminase (units/L) | |||||
| First | 258.64 ± 254.55 | 264.78 ± 233.38 | -136.99, 124.70 | - | 0.925 |
| Second | 199.97 ± 183.45 | 198.43 ± 143.76 | -86.76, 89.85 | - | 0.972 |
| Change | -58.66 ± 23.980 | -66.35 ± 191.94 | -108.69, 124.06 | 0.03 | 0.895 |
| P-value | 0.206 | 0.078 | - | - | - |
| Alanine transaminase (units/L) | |||||
| First | 165.67 ± 172.12 | 152.92 ± 119.08 | -66.55, 92.05 | - | 0.748 |
| Second | 137.24 ± 136.89 | 131.45 ± 158.82 | -73.65, 85.23 | - | 0.884 |
| Change | -28.43 ± 129.76 | -21.47 ± 128.3 | -76.11, 62.20 | 0.05 | 0.841 |
| P-value | 0.256 | 0.348 | - | - | - |
| Alkaline phosphatase (U/L) | |||||
| First | 1352.58 ± 868.72 | 1317.64 ± 763.53 | -403.19, 473.22 | - | 0.873 |
| Second | 1509.58 ± 1046.79 | 1635.21 ± 1473.83 | -810.55, 559.30 | - | 0.715 |
| Change | 156.93 ± 1211.12 | 317.56 ± 1398.59 | -861.61, 540.34 | 0.12 | 0.648 |
| P-value | 0.499 | 0.240 | - | - | - |
Abbreviations: PELD, pediatric end-stage liver disease; INR, international normalized ratio.
a Values are expressed as mean ± SD.
4. Discussion
The potential role of probiotics in modulating gastrointestinal and hepatic health has been increasingly recognized, with evidence supporting their efficacy in various conditions, including antibiotic-associated diarrhea, ulcerative colitis, celiac disease, Helicobacter pylori gastritis, and infantile colic (13, 14). Their impact on liver diseases, particularly in pediatric populations, remains an area of growing interest, with studies suggesting that probiotic supplementation may influence hepatic function through modulation of the gut-liver axis.
In this study, we evaluated the effects of L. sporogenesis on pediatric cholestatic liver disease. Our results demonstrated that PELD scores significantly decreased in the probiotic group (mean reduction: -3.98 ± 8.88, P = 0.025), whereas no significant change was observed in the placebo group. The PELD score is a critical prognostic indicator of hepatic function and the necessity for transplantation, with higher scores correlating with more severe disease progression (15). This improvement suggests that L. sporogenesis may have a beneficial effect on liver function in children with cholestatic liver disease.
Furthermore, albumin levels remained stable in the probiotic group, while a significant decline was observed in the placebo group (P = 0.009). Since albumin is a key marker of hepatic synthetic function, its maintenance in the probiotic group suggests that probiotic therapy may contribute to preserving liver function. Additionally, INR levels increased significantly in both groups, reflecting ongoing liver dysfunction. However, the increase was less pronounced in the probiotic group (0.78 ± 1.99) compared to the placebo group (1.02 ± 1.85), suggesting a potential role of L. sporogenesis in mitigating the progression of coagulopathy.
The gut-liver axis plays a crucial role in the pathophysiology of liver diseases, with the composition of the intestinal microbiota influencing hepatic inflammation, bile acid metabolism, and disease progression (16, 17). In hepatology, most research on probiotics has focused on NAFLD and obesity-related liver conditions. Evidence suggests that probiotics are effective in reducing hepatic inflammation, improving metabolic markers, and lowering hepatic venous pressure gradients (18-20).
Our findings align with these observations, indicating that L. sporogenesis may provide hepatoprotective effects by modulating the gut-liver axis in pediatric cholestatic liver disease.
Several studies have investigated the use of probiotics in pre-transplant settings, with promising results. A randomized clinical trial by Grąt et al. in cirrhotic patients awaiting liver transplantation found that probiotic supplementation significantly reduced infection rates, bilirubin concentrations, and liver enzyme levels, although it did not impact post-transplant mortality (21). Similarly, another study demonstrated that nearly 50% of cirrhotic patients receiving synbiotic therapy showed improvement in the Child-Turcotte-Pugh functional class (5). While our study did not assess post-transplant outcomes, our findings suggest that probiotics may exert beneficial effects in preserving hepatic function and delaying disease progression.
Moreover, a randomized controlled trial investigating the administration of VSL#3 in cirrhotic patients found that probiotics significantly reduced the risk of hospitalization due to hepatic encephalopathy and improved both Child-Turcotte-Pugh and MELD scores (22). However, a recent Cochrane review noted that although probiotics may help prevent overt hepatic encephalopathy, their impact on overall mortality remains inconclusive, highlighting the need for further large-scale studies (23).
Despite growing evidence in NAFLD and cirrhosis, limited research has evaluated probiotic therapy in cholestatic liver diseases. Pediatric cholestatic conditions, such as biliary atresia, remain a leading cause of liver transplantation in children, yet studies examining the effects of probiotics in this specific population are scarce (17). It has been hypothesized that dysbiosis and bile acid dysmetabolism are interconnected, with the gut microbiota playing a critical role in bile acid transformation (24-26). The ability of probiotics to regulate bile acid metabolism may contribute to their therapeutic potential in cholestatic liver disease.
Experimental models have demonstrated that Lactobacillus rhamnosus GG reduces biochemical markers of cholestasis and hepatitis in mice with bile duct obstruction or multidrug resistance protein 2 knockout. This protective effect has been attributed to the activation of the farnesoid X receptor (FXR), which regulates bile acid synthesis and enterohepatic circulation (27, 28). Our findings — particularly the improvement in PELD scores and the preservation of albumin levels — suggest that L. sporogenesis may exert similar effects in human cholestatic disease. However, further mechanistic studies are required to confirm this hypothesis.
Despite the promising results observed in this study, clinical trials evaluating probiotics in cholestatic liver disease have produced mixed findings. To date, only three randomized trials have been conducted in this population (29-31). A placebo-controlled, crossover study by Vleggaar et al. on patients with primary PSC found that probiotic therapy did not lead to significant clinical or biochemical improvements (29). This suggests that probiotics may not be effective across all cholestatic liver diseases and that their impact may vary depending on the underlying disease etiology.
However, a study by Lien et al., comparing Lactobacillus casei rhamnosus to neomycin for cholangitis prophylaxis in biliary atresia patients post-Kasai surgery, demonstrated that probiotics were as effective as antibiotic prophylaxis (30). In contrast, another study found that six months of Lactobacillus casei rhamnosus therapy did not significantly alter laboratory parameters or gut microbiota composition in biliary atresia patients (31). These findings underscore the importance of selecting the appropriate probiotic strain, treatment duration, and patient population to achieve effective clinical outcomes.
4.1. Conclusions
This randomized, triple-blind, placebo-controlled trial investigated the effects of L. sporogenesis in children with cholestatic liver disease. Although no statistically significant difference in PELD scores was observed between the probiotic and placebo groups, the intervention group demonstrated a notable reduction in PELD scores following treatment.
Additionally, albumin levels remained stable in the probiotic group, while a significant decline was observed in the placebo group, suggesting a potential protective effect of probiotics on hepatic synthetic function. These findings highlight the potential of L. sporogenesis as a complementary nutritional therapy in the management of cholestatic liver disease in children. Further studies with larger sample sizes, extended intervention durations, and detailed mechanistic investigations are essential to validate these results and to better understand the therapeutic role of probiotics in pediatric liver diseases.
4.2. Limitations
As with any study, our research has certain limitations. One of the primary constraints is the relatively small sample size (56 patients), which may limit the statistical power of our findings. However, given the rarity of cholestatic liver diseases in children, our study still provides valuable insights, and our sample size is larger than that of many previous studies on this topic. Nevertheless, future research involving larger cohorts is necessary to confirm our results.
Another limitation is the short intervention duration (4 weeks), which may not be sufficient to observe the full clinical effects of probiotics. While our study aimed to assess early responses, longer intervention periods (e.g., 3 to 6 months) would provide a more comprehensive understanding of the sustained benefits of L. sporogenesis in this population.
Additionally, our study did not include an analysis of gut microbiota changes following probiotic administration, which could have provided mechanistic insights into the role of probiotics in cholestatic liver diseases. Future studies should incorporate microbiota profiling to explore this aspect further. Similarly, we did not evaluate inflammatory cytokines or other biological markers related to the gut-liver axis. Investigating these markers in future research would help elucidate the underlying pathways through which probiotics exert their effects on liver function and inflammation.
Despite these limitations, our study offers meaningful contributions to the growing body of research on probiotics in pediatric cholestatic liver diseases. Further investigations with larger sample sizes, extended follow-up durations, and additional mechanistic analyses are warranted to build upon our findings.