1. Background
Toxoplasmosis is a prevalent global ailment impacting roughly one-third of the world’s population. This disease is triggered by the coccidian parasite Toxoplasma gondii, which relies on cats as its definitive host, whereas warm-blooded animals serve as intermediate hosts (1-3). Toxoplasma gondii can be transmitted through several routes. Key transmission pathways include ingesting oocysts from contaminated water, soil, or vegetables, as well as consuming undercooked or raw meat containing tissue cysts. Another major route is vertical transmission from mother to fetus during pregnancy (4). While transmission of T. gondii during embryogenesis is infrequent, occurring in less than 6% of cases, it can have severe consequences for the fetus. In contrast, transmission rates during the third trimester are significantly higher, ranging from 60% to 81%. Despite this, newborns may not always show symptoms (5).
It is crucial to recognize that primary T. gondii infection during pregnancy can lead to severe consequences, including spontaneous abortion, neonatal death, and congenital disorders such as cerebral and visual abnormalities. In early life, the infection profoundly affects neurological development, primarily impacting the central nervous system (CNS) (3, 6). Diagnosing T. gondii infection in humans is typically done through serological methods that detect anti-Toxoplasma IgG and IgM antibodies (7). The IgM antibodies can usually be identified about two weeks after infection and tend to decline to undetectable levels within a few months. In contrast, IgG antibodies become detectable approximately 14 days after the first positive IgM test and remain present indefinitely (8).
All pregnant women should undergo initial T. gondii screening early in pregnancy. For those with negative initial results, subsequent screenings are recommended monthly or at least once per trimester to enable early detection of infection and prompt treatment (9). The variation in prevalence from one region to another suggests that factors such as cultural practices, climate, and the presence or absence of cats play a significant role in influencing prevalence rates (4). A comprehensive understanding of prevailing and emergent T. gondii infection rates within a nation’s human population is of paramount importance. This data serves as a cornerstone for conducting precise risk assessments, implementing effective public health education initiatives, and making informed policy determinations (10).
2. Objectives
The present study aimed to evaluate the seroprevalence of T. gondii and associated risk factors among pregnant women in the Beni Mellal region of Morocco.
3. Methods
3.1. Study Design and Study Area
The study was conducted from December 1, 2021, to March 18, 2024, in Beni Mellal province, located in central Morocco within the Beni Mellal-Khenifra region. This region is known for its continental climate, featuring extremely hot summers with temperatures surpassing 40°C and cold winters with temperatures dropping to 0°C. The research was an institution-based cross-sectional study conducted at health facilities providing attending antenatal care (ANC) services to pregnant women.
3.2. Population and Data Collection
The optimal sample size was determined using the following assumptions: n = z2 × P (1 - P)/d2 where n is the sample size, P = 0.5 (no previous estimate of prevalence of T. gondii in Beni Mellal province), d is the desired marginal error = 0.075, and z is the confidence level 95% = 1.96, resulting in a sample size of 171 participants. A structured questionnaire was developed to explore known risk factors for toxoplasmosis, including sociodemographic data such as age, educational level, residence, and number of children. Additionally, gyneco-obstetrical history and toxoplasmosis serological results were collected. Serological analyses were performed using immunoenzymatic tests, specifically enzyme linked fluorescent assay (ELFA) to detect IgG antibodies against T. gondii (VIDAS Toxo IgGII, bioMerieux, France). Testing was conducted following the manufacturer’s guidelines, with IgG levels < 4 IU/mL considered negative and levels > 8 IU/mL deemed positive. The study also evaluated participants’ knowledge of toxoplasmosis and collected information on behavioral and lifestyle factors, including contact with cats, consumption of raw or undercooked meat, intake of raw or unwashed fruits and vegetables, handwashing practices after handling raw meat, exposure to garden soil, and drinking untreated water.
3.3. Inclusion Criteria
Included pregnant women attending antenatal consultations who underwent routine toxoplasmosis serological testing and were residents in the Beni Mellal-Khenifra region. Participants were informed about the research objectives and provided informed consent to participate in the study.
3.4. Ethical Considerations
Permission was obtained from Morocco’s Ministry of Health and Social Protection to collect the data (Ref. N°190/2020). The principle of anonymity was maintained throughout the entire process.
3.5. Statistical Analysis
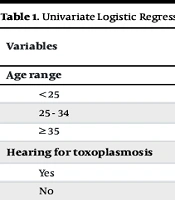
Data collected through the questionnaire were entered into SPSS Statistics (version 21) for analysis. Descriptive statistics were utilized to summarize the data, with statistical significance set at a P-value of < 0.05. Initially, univariate logistic regression was conducted to identify significant variables for inclusion in the multivariate logistic regression analysis. Odds ratios (OR) and 95% confidence intervals (CI) were used to determine the strength of any observed associations.
4. Results
A total of 171 pregnant women, aged 18 to 44 years (mean age, 28.94 years), participated in the study. The normality of the age data was assessed using the Kolmogorov-Smirnov test, which indicated that the age distribution was normal (P = 0.155). Among the participants, 18% were illiterate, and 90% were housewives. Additionally, 95% of the participants were from urban areas. Half of the women (50.3%) had fewer than three children, and 35.7% were primigravida. Regarding gestational age, 33.9% were in the first trimester, 45.6% in the second trimester, and 20.5% in the third trimester. For 85.4% of the women, this was their first serological test for toxoplasmosis.
Among the 171 women surveyed, 39.2% (67/171) (95% CI: 31.6 - 46.2) tested positive for T. gondii-specific IgG antibodies, indicating a previous infection. The mean IgG concentration in those who tested positive was 76.03 IU/mL (range: 16.60 - 146.92). However, the Kolmogorov-Smirnov test indicated that the distribution of IgG concentration data was not normal (P = 0.000). Merely 4.1% of the participants underwent repeat toxoplasmosis serology during their pregnancy. While the majority (92.4%) had received obstetrical ultrasound examinations, which were reported as normal.
Regarding obstetrical history, 13.5% had experienced a miscarriage, 3% had a fetal death in utero, 2% had given birth to a baby with a congenital malformation, and 1.8% had a neonatal death. The reported congenital malformations included congenital heart disease, hydrocephalus, and trisomy 21. Despite having previously undergone serological testing, only 21.1% of the women had heard of toxoplasmosis. Furthermore, all women identified contact with cats as a potential source of infection. Additionally, 11.7% reported the occurrence of cats in their environment. Within this group, 60% did not handle cat litter, 10% cleaned the litter without gloves, and 30% delegated this task to another family member.
Among these women, 9.4% consumed undercooked meat, 84.8% ate raw vegetables, and only 2.3% drank untreated water. The vast majority of respondents reported that they washed their hands prior to eating and after handling fresh fruit and vegetables. However, 13.5% admitted to not washing their hands after handling raw meat. Univariate analysis revealed significant associations between Toxoplasma seropositivity and hearing about the disease (reduced risk) and cat ownership (increased risk). Good hygiene practices, such as handwashing, showed a trend towards reduced risk, while age, dietary factors (undercooked meat, raw vegetables), and drinking untreated water were not significantly associated with infection, as shown in Table 1.
| Variables | Positive Serology | Total | P-Value | OR | CI (95%) | |
|---|---|---|---|---|---|---|
| Yes; 67 (39.2) | No; 104 (60.8) | |||||
| Age range | ||||||
| < 25 | 14 (28) | 36 (72) | 50 (29.2) | 0.596 | 1.286 | 0.508 - 3.252 |
| 25 - 34 | 41 (48.2) | 44 (51.8) | 85 (49.7) | 0.133 | 0.537 | 0.216 - 1.413 |
| ≥ 35 | 12 (33.3) | 24 (66.7) | 36 (21.1) | 0.051 | 1.00 | Ref |
| Hearing for toxoplasmosis | 0.001 b | |||||
| Yes | 23 (63.9) | 13 (36.1) | 36 (21.1) | 1.00 | Ref | |
| No | 44 (32.6) | 91 (67.4) | 135 (78.9) | 3.659 | 1.695 - 7.898 | |
| Owner of a cat | 0.001 b | |||||
| Yes | 15 (75) | 5 (25) | 20 (11.7) | 0.175 | 0.060 - 0.509 | |
| No | 52 (34.4) | 99 (65.6) | 151 (88.3) | 1.00 | Ref | |
| Consumption of undercooked meat | 0.052 | |||||
| Yes | 10 (62.5) | 6 (37.5) | 16 (9.4) | 0.342 | 0.120 - 1.011 | |
| No | 57 (36.8) | 98 (63.2) | 155 (90.6) | 1.00 | Ref | |
| Handwashing after contact with raw meat | 0.002 b | |||||
| Yes | 51 (34.5) | 97 (65.6) | 148 (86.5) | 1.00 | Ref | |
| No | 16 (69.6) | 7 (30.4) | 23 (13.5) | 0.230 | 0.089 - 0.595 | |
| Consumption of raw vegetables | 0.935 | |||||
| Yes | 57 (39.3) | 88 (60.7) | 145 (84.8) | 0.965 | 0.409 - 2.275 | |
| No | 10 (38.5) | 16 (61.5) | 26 (15.2) | 1.00 | Ref | |
| Drinking untreated water | 0.999 | |||||
| Yes | 4 (100) | 0 (0) | 4 (2.3) | 0.000 | 0.000 | |
| No | 63 (37.7) | 104 (62.3) | 167 (97.7) | 1.00 | Ref | |
| Proper hand washing after contact with soil-soiled vegetables and fruits | 0.029 b | |||||
| Yes | 51 (34.5) | 97 (65.5) | 148 (86.5) | 1.00 | Ref | |
| No | 16 (69.6) | 7 (30.4) | 23 (13.5) | 0.219 | 0.056 - 0.858 | |
Univariate Logistic Regression Analysis of the Variables Associated with the Seroprevalence of Toxoplasmosis Among the Sample (n = 171) a
These four factors were included in a multivariate analysis, which showed that awareness of toxoplasmosis was significantly associated with a lower risk of infection, suggesting that public health campaigns may be effective in reducing infection rates. Conversely, cat ownership significantly increased the risk, aligning with the known association between cat feces and Toxoplasma transmission. Additionally, good hygiene practices, including handwashing after handling raw meat or contaminated produce, were associated with a lower risk, although this association was not always statistically significant in all models, as shown in Table 2.
| Variables | Adjusted | P-Value | |
|---|---|---|---|
| OR | 95% CI | ||
| Hearing for Toxoplasmosis | 0.001 | ||
| Yes | 1 | Ref | |
| No | 4.110 | 1.806 - 9.351 | |
| Owner of a cat | 0.001 | ||
| No | 0.141 | 0. 046 - 0.430 | |
| Yes | 1 | Ref | |
| Handwashing after contact with raw meat | 0.068 | ||
| Yes | 1 | Ref | |
| No | 0.318 | 0.093 - 1.086 | |
| Proper hand washing after contact with soil, soiled vegetables and fruits | 0.333 | ||
| Yes | 1 | Ref | |
| No | 0.422 | 0.073 - 2.419 | |
Multivariate Logistic Regression Analysis of the Variables Associated with the Seroprevalence of Toxoplasmosis Among Pregnant Women (n = 171)
5. Discussion
The study found an overall seroprevalence of T. gondii infection to be 39.2% (95% CI: 31.6 - 46.2), consistent with the 39.7% seroprevalence reported in eastern Morocco (11). However, this prevalence is higher than the 26.28% reported in a recent study conducted in Marrakech (12), yet lower than the 43% observed in Rabat (13). In contrast, significantly higher seroprevalence rates have been reported in several African countries, with 85.3% in Cameroon (14) and 92.5% in Ghana (15). Conversely, lower prevalences have been documented in Vietnam (5.8%) (16), the United Kingdom (9.1%) (17), and Japan (10.3%) (18). The variation in T. gondii prevalence across different countries, and even within the same country, can be attributed to factors that influence oocyst sporulation and survival in the environment. Environmental and geographical characteristics play a crucial role in oocyst persistence. Infections tend to be more common in hot climates and low-lying areas compared to cold climates and mountainous regions, as well as in humid environments versus dry ones (13, 19).
Primary prevention of congenital toxoplasmosis focuses on preventing maternal infection through counseling and education for women before and during early pregnancy to minimize their exposure risk. Secondary prevention strategies involve maternal serological screening, fetal diagnosis, and potential interventions such as in-utero treatment or, in severe cases, consideration of pregnancy termination (20). The initial diagnostic approach usually involves serologic testing to detect IgG and IgM antibodies. However, distinguishing between primary and chronic infections can be challenging, as interpreting IgG and IgM results often proves complex (21). To improve the accuracy of interpreting results, it is recommended to collect two serological samples spaced three weeks apart, with an IgM test performed on the initial sample. Analyzing antibody dynamics across these successive samples should be done in the same laboratory, using consistent techniques and within the same series of tests (22). The IgM antibodies appear earlier after infection than IgG and usually disappear faster. However, both can persist beyond the acute phase. To distinguish recent infections, avidity tests are crucial. Avidity measures the strength of IgG antibody binding to the parasite, helping differentiate recent infections with lower avidity from past infections with higher avidity (23).
In our current study, we did not identify a significant association between age and T. gondii infection. These findings align with similar results from Serbia (24) and Italy (25). However, a cross-sectional study in Norway reported a higher prevalence of toxoplasmosis among women aged 40 and above (OR: 2.65, 95% CI: 1.30 - 5.42) (26). It’s also important to note that the risk of fetal transmission increases with the age of the pregnancy, as the placenta becomes more permeable over time (27, 28). Additionally, in our study, about 45.6% of the pregnant women were in their second trimester. We did not find any association between T. gondii infection and adverse outcomes of previous pregnancies, such as miscarriage, fetal death in utero, congenital abnormalities, or stillbirth. This result is consistent with findings from a study conducted in Belgrade (24). However, our findings differ from other research that has identified an association between T. gondii infection and pregnancy outcomes (29). A meta-analysis on the risk of vertical transmission of T. gondii and adverse pregnancy outcomes concluded that T. gondii infection can indeed lead to pregnancy complications (30).
Furthermore, our study did not identify a connection between the consumption of undercooked meat and T. gondii infection, which is consistent with findings from a study in Ghana (15). On the other hand, multiple studies have shown an association between the consumption of various meats and recent T. gondii infection (31-33). Additionally, handling meat has been linked to T. gondii infection in other research (34, 35). While our study did not find an association between fresh vegetable consumption and toxoplasmosis infection, numerous studies worldwide have documented the contamination of fresh vegetables with T. gondii oocysts (36-39).
Our research further supports the notion of a link between close contact with cats and susceptibility to toxoplasmosis. This aligns with findings from a study in Brazil involving 492 pregnant women, where having a cat in the home was associated with a higher likelihood of T. gondii infection (40). Cats are the primary source of oocysts that can contaminate soil, and even ingesting a single bradyzoite can lead to the shedding of millions of oocysts (41). Remarkably, a large-scale study in China revealed that T. gondii DNA is prevalent in soil samples from schools, parks, farms, and coastal beaches (42). Our univariate analysis identified inadequate handwashing after soil contact as a risk factor for toxoplasmosis, which is consistent with findings from a similar study in Egypt (43). Additionally, awareness about toxoplasmosis was found to be a protective factor against T. gondii infection. Conversely, a study of blood donors in Egypt highlighted that insufficient knowledge about the disease was a risk factor for toxoplasmosis (43). This suggests that awareness campaigns about the disease may be effective in reducing infection rates.
Moreover, in our study, the majority of participants consumed well-treated water, which prevented us from establishing a link between water consumption and the risk of contracting toxoplasmosis. However, several other studies have found an association between the consumption of untreated water and the development of toxoplasmosis in both humans and animals (32, 44, 45). Lastly, our findings identified contact with cats and lack of awareness about toxoplasmosis as significant risk factors for infection. In contrast, a Brazilian study on the Island of Fernando de Noronha found that consuming well or rainwater and consuming game meat were associated with increased infection risk (46). These contrasting findings likely reflect differences in cultural practices and environmental factors influencing exposure pathways in these distinct geographical locations.
5.1. Conclusions
The present study found that 60.02% of pregnant women in the area were at risk of severe T. gondii infection. Presently, there are no legal requirements in our country for public education on reducing T. gondii exposure, revealing a significant gap in preventive measures. It is crucial for health care providers to address this issue. We recommend that pregnant women rigorously adhere to thorough hygiene practices to prevent T. gondii infection. This includes meticulous handwashing with soap and water after handling raw meat, gardening, changing cat litter, and any contact with soil. Safe food handling practices are paramount, with an emphasis on proper cooking of meat. The study also recommends that future research investigate the potential contamination of water, vegetables, and fruits by T. gondii and explore how these factors could be incorporated into prevention strategies.
5.2. Limitations of the Study
This study has several limitations. Firstly, the cross-sectional design precludes the establishment of causal relationships between Toxoplasma infection and the identified risk factors. Secondly, the study population comprised pregnant women ANC, which may not fully reflect the characteristics of the general pregnant population in the country. Thirdly, a follow-up serological study to confirm seroconversion in IgG-negative participants was not conducted, and IgG avidity tests were not performed in these groups, which could have provided more precise information on the timing of infection.