1. Background
Common pulmonary diseases in neonates include bronchopulmonary dysplasia (BPD), pneumothorax, atelectasis, pneumonia of the newborn (PN), neonatal pulmonary hemorrhage (NPH), acute respiratory distress syndrome (ARDS), neonatal respiratory distress syndrome (NRDS), meconium aspiration syndrome (MAS), and transient tachypnea of the newborn (TTN). Clinical history, physical examination, arterial blood gas measurement, chest radiography, and computed tomography (CT) scans are all important components of traditional diagnostic methods. However, neonates who undergo recurrent CT and chest radiography exams are at risk of radiation exposure and face inconvenience (1). Due to its portability, real-time imaging capabilities, high repeatability, affordability, and lack of ionizing radiation, lung ultrasound (LUS) has become a valuable diagnostic and monitoring tool (2-4). Lung ultrasound is increasingly used as it has demonstrated potential in managing several lung conditions (2, 3). Although extensive research has investigated LUS findings in specific infant lung illnesses, a comprehensive comparison of ultrasonographic features across these prevalent conditions is lacking (5-9).
2. Objectives
The present study aimed to describe and contrast the ultrasonographic characteristics of these common infant lung disorders to improve diagnostic precision.
3. Methods
3.1. Study Population
From March 2018 to January 2024, a cross-sectional study was conducted at the obstetrics and neonatal departments of Dehong People’s Hospital, affiliated with Kunming Medical University. The ethics committee of the hospital approved the study protocol (2018DY008), and informed consent was obtained from each participant’s guardian.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) Children diagnosed by clinical combined chest X-ray and LUS diagnosis, with consistent results from both methods; (2) age ≤ 1 day, with LUS and chest radiography performed within 15 minutes of each other; (3) no fetal malformations or developmental abnormalities; (4) no medical disputes between the hospital and the guardians; and (5) parental consent to participate. Neonates with skin disorders, skin lesions, or other conditions preventing an LUS examination were excluded, as were those with suspected or confirmed HIV or syphilis infection. Standardization of data collection methods was ensured to reduce observer bias.
3.3. Research Design and Information Gathering
Between March 2019 and January 2024, 466 neonates who met the inclusion criteria were enrolled in this descriptive study. As controls, 97 healthy newborns from the obstetrics and neonatal departments were included. A thorough characterization of the ultrasonographic findings of prevalent newborn lung disorders was conducted. Ultrasonographic parameters, such as the frequency and duration of B-lines in the TTN group stratified by gestational age, were compared between the TTN group and controls.
3.4. Lung Ultrasound Examination
A Mindray M5T portable ultrasound system (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) with a high-frequency linear array probe (≥ 9 MHz) was used to perform LUS exams (2-4). One focus point was placed at the pleura-lung interface, and the scanning depth was adjusted to 4 - 6 cm. Spatial compounding was turned off. Board-certified neonatologists conducted and interpreted every evaluation. Sequential longitudinal and transverse scans of each lung region (bilaterally) were used as part of a systematic strategy. Throughout the scanning process, the probe was kept perpendicular to the ribs or pleura. Typically, the lungs are divided into three regions: Anterior, lateral, and posterior, with the anterior axillary line and posterior axillary line as the boundaries. Therefore, the lungs on both sides are divided into six regions for continuous scanning. When the weight of the examinee is ≥ 3 kg, each lung is divided into upper and lower lung fields with the line connecting the two nipples as the boundary, dividing both lungs into 12 areas for continuous scanning. Tables 1 - 4 present the results of the LUS.
| Ultrasonographic Finding | TTN (n = 88) | NRDS (n = 69) | ARDS (n = 29) | MAS (n = 57) | PN (n = 89) | NPH (n = 6) | BPD (n = 20) | Atelectasis (n = 3) | Pneumothorax (n = 8) | Control (n = 97) |
|---|---|---|---|---|---|---|---|---|---|---|
| B-lines | 71 (80.7) | 56 (81.2) | 24 (82.8) | 49 (86.0) | 77 (86.5) | 6 (100) | 12 (60.0) | 2 (66.7) | 0 (0) | 89 (91.8) |
| AIS/dense B-lines/white lung | 83 (94.3) | 11 (15.9) | 4 (13.8) | 11 (19.3) | 19 (21.3) | 3 (50) | 2 (10.0) | 1 (33.3) | 0 (0) | 0 (0) |
| Pulmonary point signs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (75) | 0 (0) |
| Double lung points | 72 (81.8) | 31 (44.9) | 13 (44.8) | 30 (52.6) | 48 (53.9) | 2 (33.3) | 3 (15.0) | 0 (0) | 0 (0) | 0 (0) |
| Abnormal pleural line | 88 (100) | 69 (100) | 29 (100) | 57 (100) | 89 (100) | 6 (100) | 12 (60.0) | 1 (33.3) | 0 (0) | 0 (0) |
| A-line disappearance/reduction | 88 (100) | 69 (100) | 29 (100) | 57 (100) | 89 (100) | 6 (100) | 12 (60.0) | 1 (33.3) | 0 (0) | 0 (0) |
| Lung consolidation/etc. | 0 (0) | 69 (100) | 29 (100) | 57 (100) | 89 (100) | 6 (100) | 20 (100) | 1 (33.3) | 0 (0) | 0 (0) |
| Abnormal lung slip | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (100) | 0 (0) |
| Stratospheric sign | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (100) | 0 (0) |
| Beach sign | 88 (100) | 69 (100) | 29 (100) | 57 (100) | 89 (100) | 6 (100) | 20 (100) | 3 (100) | 0 (0) | 97 (100) |
| Pleural effusion | 17 (19.3) | 13 (18.8) | 3 (10.3) | 9 (15.8) | 19 (21.3) | 5 (83.3) | 2 (10.0) | 0 (0) | 0 (0) | 0 (0) |
| Spared areas | 28 (31.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Ultrasonographic Findings in Neonatal Lung Diseases a
| Ultrasonographic Finding | TTN (n = 88) | Controls (n = 97) | χ2 or Fisher’s Exact Test | P-Value |
|---|---|---|---|---|
| White lung | 29 (32.95) | 0 (0.00) | 37.91 | < 0.01 |
| Dense B-lines | 62 (70.45) | 0 (0.00) | 102.79 | < 0.01 |
| AIS | 83 (94.32) | 0 (0.00) | 165.94 | < 0.01 |
| B-lines artifacts | 71 (80.68) | 97 (100.00) | 20.63 | < 0.01 |
| Double lung points | 72 (81.82) | 0 (0.00) | 129.93 | < 0.01 |
| Pleural effusion | 17 (19.32) | 1 (1.03) | 17.57 | < 0.01 |
| Lung consolidation | 0 (0.00) | 0 (0.00) | - | - |
| Abnormal pleural line/A-lines | 88 (100.00) | 0 (0.00) | 185.00 | < 0.01 |
Comparison of Ultrasonographic Findings Between Neonates with Transient Tachypnea of Newborn and Controls a
| Gestational Age (wk) | TTN (n = 88) | Controls (n = 97) | χ2 or Fisher’s Exact Test | P-Value |
|---|---|---|---|---|
| ≤ 32 | 28(31.82) | 8(8.25) | 16.36 | < 0.01 |
| ≤ 34 | 40(45.45) | 19(19.59) | 4.98 | < 0.05 |
| ≤ 37 | 14(15.91) | 28(28.86) | 4.41 | < 0.05 |
| ≤ 42 | 6(6.82) | 38(39.18) | 26.65 | < 0.01 |
| ≥ 42 | 0(0.00) | 4(4.12) | 2.02 | > 0.05 |
Frequency of B-lines in Neonates with Transient Tachypnea of Newborn Stratified by Gestational Age a
| Gestational Age (wk) | TTN (n = 88) | Controls (n = 97) | t-Test or Mann-Whitney U | P-Value |
|---|---|---|---|---|
| ≤ 32 | 221.75 ± 42.81 | 143.23 ± 29.97 | 14.557 | 0.000 |
| ≤ 34 | 177.66 ± 32.17 | 103.27 ± 23.74 | 18.005 | 0.000 |
| ≤ 37 | 110.45 ± 22.69 | 84.00 ± 19.77 | 8.471 | 0.000 |
| ≤ 42 | 77.09 ± 17.55 | 48.12 ± 11.67 | 13.332 | 0.000 |
| ≥ 42 | 67.28 ± 15.41 | 38.48 ± 9.21 | -39.184 | 0.000 |
Duration of B-lines (Hours, Mean ± SD) in Neonates with Transient Tachypnea of Newborn Stratified by Gestational Age
3.5. Ultrasound Parameters Seen
The interface between the visceral pleura and the lung parenchyma is represented by the pleural line, a hyperechoic line resulting from the tissues’ different acoustic impedances. In healthy lungs, the pleural line is continuous, crisp, and smooth. When the probe is positioned perpendicular to the ribs, the horizontal movement of the visceral pleura relative to the parietal pleura during respiration is known as lung sliding, visible as a dynamic gliding motion on real-time ultrasound.
- A-lines: When the ultrasonic beam is perpendicular to the pleural surface, reverberation artifacts cause these evenly spaced, horizontal hyperechoic lines to appear parallel to and directly below the pleural line. Their intensity progressively diminishes with increasing depth.
- B-lines: These are hyperechoic, vertically oriented lines that run from the pleural line to the base of the lung. During breathing, they move in tandem with the pleural line sliding.
- Confluent B-lines: These are B-lines so closely packed together in an intercostal area that they are indistinguishable from one another, with the rib shadow still discernible. Confluent B-lines in at least two successive intercostal gaps within a single scan region indicate AIS.
- Dense B-lines: B-lines are referred to as dense B-lines when they are so numerous that the rib shadow is nearly hidden. The appearance of the lung when dense B-lines are seen in both scan regions is referred to as "white lung".
- Spared areas: Typically aerated lung parenchyma areas at least one intercostal space in size, encircled by consolidated lung tissue, with discernible pleural line and A-lines within the lung island.
- Lung consolidation: The appearance of lung tissue on ultrasonography that mimics the liver’s echogenicity, or "hepatization".
- Lung pulsation: Real-time ultrasound shows a discernible pulsation of consolidated lung tissue in rhythm with the cardiac cycle, especially when the consolidation is considerable and near the cardiac boundary.
- Fragmentation sign: The blurred line separating consolidated and aerated lung tissue, manifesting as an uneven or broken echotexture.
- Ground-glass opacity: A modest form of consolidation appearing as a hazy, uniform lung pattern on ultrasonography with little bronchial air trapping.
- Snowflake sign: A pattern showing substantial consolidation characterized by a heterogeneous, punctate echotexture with apparent bronchial air trapping.
- Pulmonary points: Defined locations where lung sliding occurs sporadically during respiration, identified by real-time ultrasonography.
- Double lung points: Distinct demarcation points between the upper and lower lung fields on LUS, indicating differences in the type or amount of illness.
- Stratospheric sign/barcode sign: When lung sliding is absent, parallel lines in M-mode ultrasound replace the granular echotexture typically seen under the pleural line, indicating pneumothorax.
- Beach sign/sandpaper sign: An echotexture that is uniformly granular and resembles a beach seen in M-mode ultrasonography, indicating normal lung sliding and ruling out pneumothorax.
- Pleural effusion: The accumulation of excess fluid in the pleural space.
3.6. Statistical Analysis
Data were analyzed using SPSS software, version 29.0. The χ2 test or Fisher’s exact test, where applicable, was used to evaluate intergroup differences in proportions for categorical variables, which are expressed as percentages (Tables 2 and 3). Continuous variables with a normal distribution are represented as mean ± standard deviation (SD), and the paired t-test was used to assess differences across groups (Table 4). Statistical significance was defined as a P-value of less than 0.05.
4. Results
4.1. Overview of Research Participants
Out of 471 newborns, 1 was excluded due to HIV, 2 due to syphilis, and 2 had disputes with the hospital. Ultimately, 466 newborns were included in the study. Based on diagnostic categorization [TTN, NRDS, ARDS, MAS, PN, NPH, BPD, atelectasis, pneumothorax, and controls], Table 5 shows the baseline characteristics of the 466 newborns. Among the 466 study participants, Table 5 lists 97 cases in the normal control group, 88 cases of TTN, 69 cases of NRDS, 29 cases of ARDS, 57 cases of MAS, 89 cases of PN, 6 cases of NPH, 20 cases of BPD, 3 cases of neonatal atelectasis, and 8 cases of neonatal pneumothorax.
| Characteristics | TTN (n = 88) | NRDS (n = 69) | ARDS (n = 29) | MAS (n = 57) | PN (n = 89) | NPH (n = 6) | BPD (n = 20) | Atelectasis (n = 3) | Pneumothorax (n = 8) | Control (n = 97) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender (male/female) | 49/39 | 36/33 | 16/13 | 30/27 | 47/42 | 4/2 | 10/10 | 1/2 | 3/5 | 51/46 |
| Gestational age (wk) | 31.68 ± 2.03 | 30.75 ± 2.34 | 33.55 ± 3.21 | 39.51 ± 3.36 | 36.86 ± 2.96 | 32.11 ± 1.53 | 31.15 ± 1.81 | 38.37 ± 3.52 | 33.14 ± 3.17 | 38.36 ± 3.12 |
| Age at ultrasound diagnosis (h) | 19.75 ± 3.20 | 3.37 ± 1.05 | 25.44 ± 3.33 | 19.47 ± 8.93 | 123.79 ± 24.56 | 70.88 ± 21.15 | 696.12 ± 88.21 | 68.36 ± 13.56 | 51.91 ± 12.81 | 28.31 ± 7.34 |
| Birth weight (kg) | 1.98 ± 0.54 | 1.69 ± 0.53 | 2.83 ± 0.36 | 3.89 ± 0.77 | 3.15 ± 0.44 | 2.74 ± 0.36 | 1.79 ± 0.52 | 3.10 ± 0.75 | 2.13 ± 0.46 | 3.15 ± 0.62 |
| Preterm/term | 82/6 | 67/2 | 21/8 | 0/57 | 60/27 | 4/2 | 20/0 | 1/2 | 6/2 | 55/42 |
| Vaginal/cesarean | 32/56 | 31/38 | 15/14 | 32/25 | 42/47 | 2/4 | 7/13 | 1/2 | 5/3 | 45/52 |
Characteristics of Neonates by Diagnostic Group a
4.2. Incidence Rates and Ultrasound Imaging Features of Common Pulmonary Disorders in Neonates
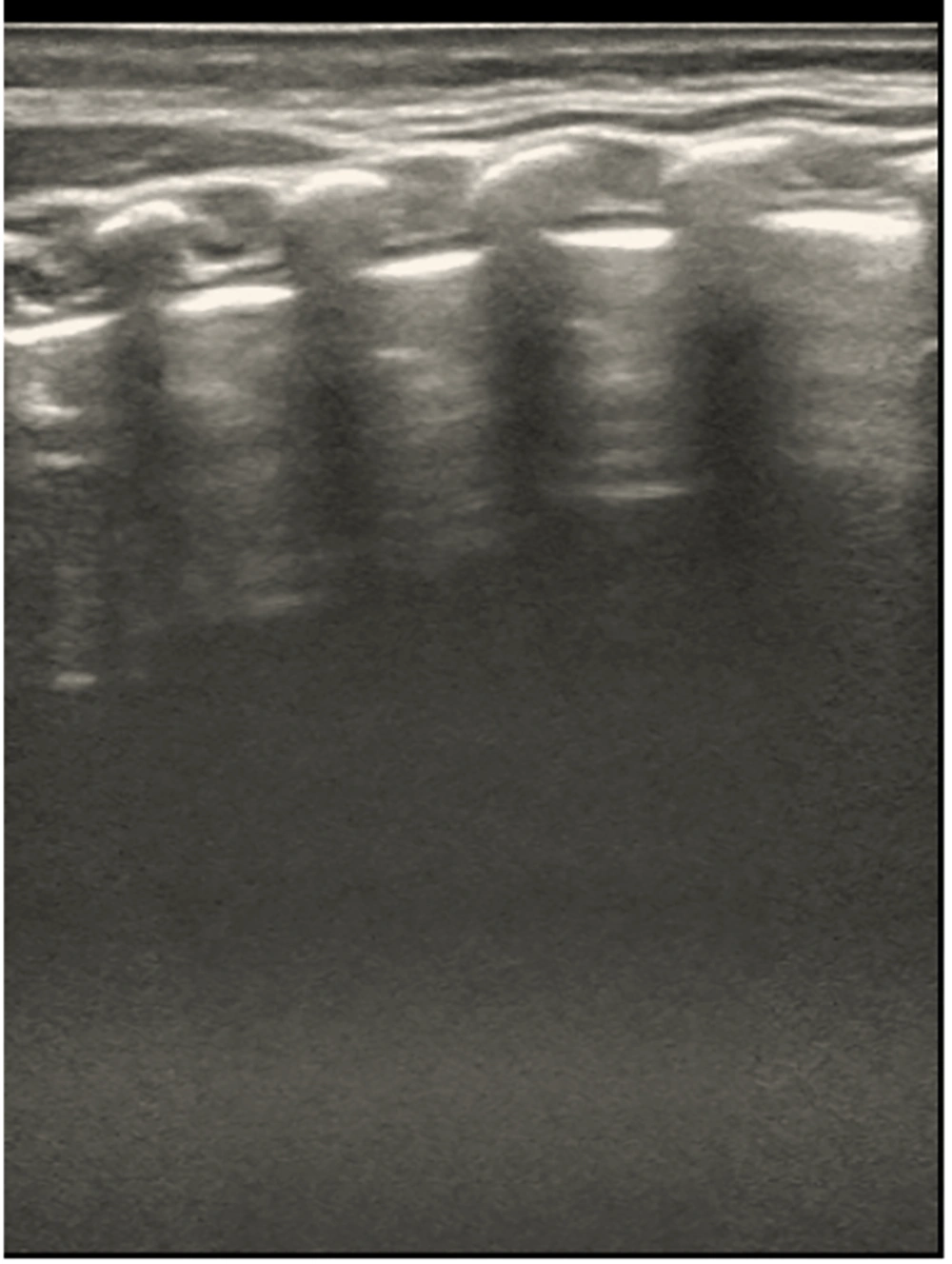
Normal newborn lungs on ultrasound display lung sliding, equidistant and parallel arrangement of A-lines and pleural lines, and smooth, clear, and regular pleural and A-lines. Normal lung imaging shows a classic beach sign on M-mode ultrasound, with no pulmonary consolidation or alveolar interstitial syndrome.
In TTN, ultrasound imaging characteristics include pleural line abnormalities (100.00%), disappearance or reduction of A-lines (100.00%), beach sign (100.00%), B-lines (80.68%), AIS and/or dense B-lines and/or white lung (94.32%), bipulmonary foci (81.82%), pleural effusion (19.32%), and lung islands (31.82%). There are no indications of pneumothorax or pulmonary consolidation.
For NRDS, ARDS, MAS, and PN, ultrasound features include lung consolidation, fragmentation, ground-glass sign, snowflake sign, and pulmonary pulsations (100.00%), along with the beach sign (100.00%). These are also characteristics of NPH and BPD, with common features including lung consolidation or hepatoid transformation. B-lines, AIS, dense B-lines, multiple lung points, aberrant pleural lines, disappearance or reduction of A-lines, and pleural effusion are all possible.
Neonatal atelectasis ultrasonography reveals pulmonary consolidation, but no pleural effusion or bilateral lung points are observed. Neonatal pneumothorax is characterized by abnormal or nonexistent lung sliding (100.00%), stratospheric sign (100.00%), and pulmonary spot (75.00%) on ultrasonography.
Refer to Figures 1 -7 and Table 1 for detailed imaging features.
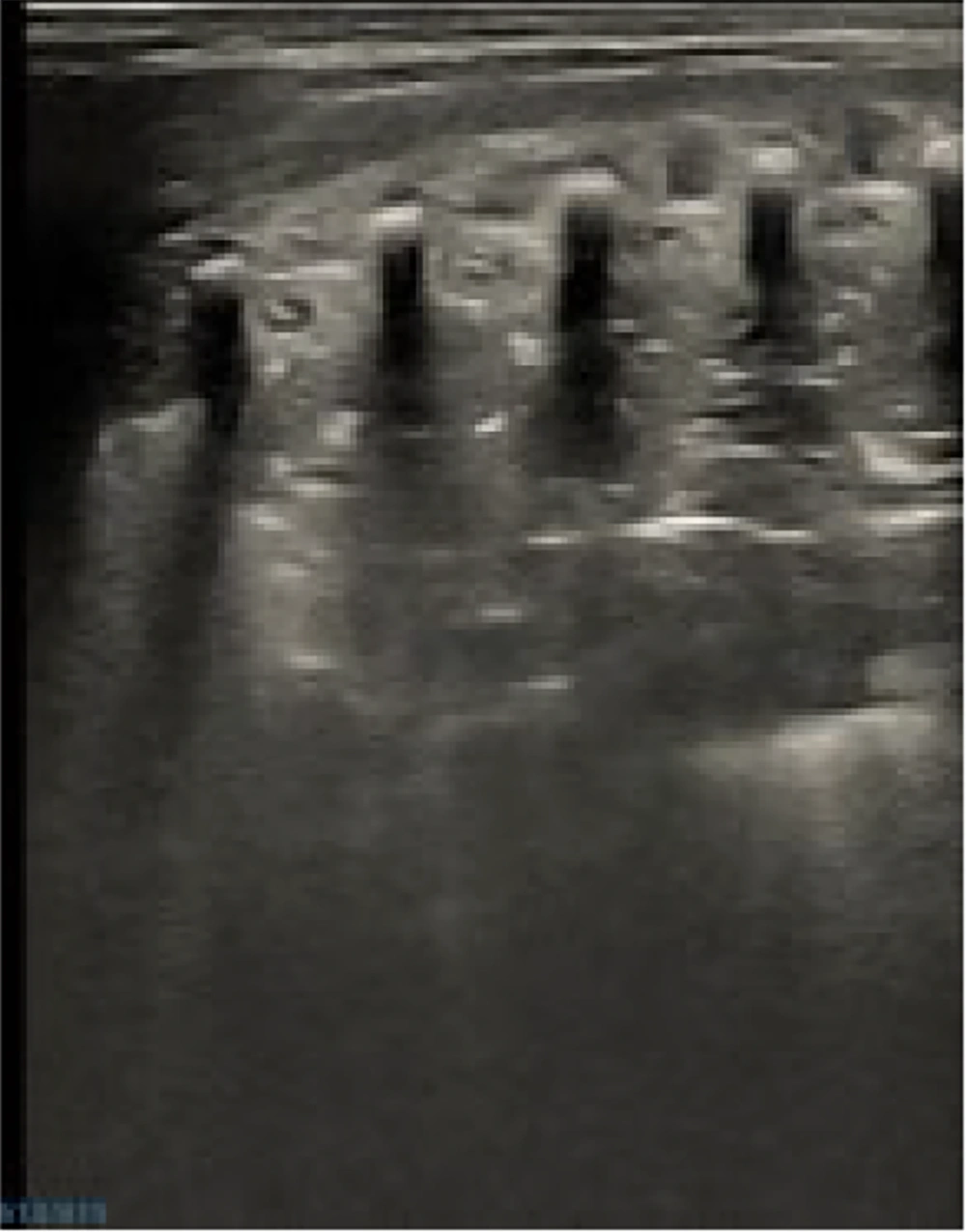
Representative ultrasound image of normal newborn lung. Normal lung aeration is indicated by the image’s parallel, evenly spaced A-lines and smooth, clear pleural lines. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
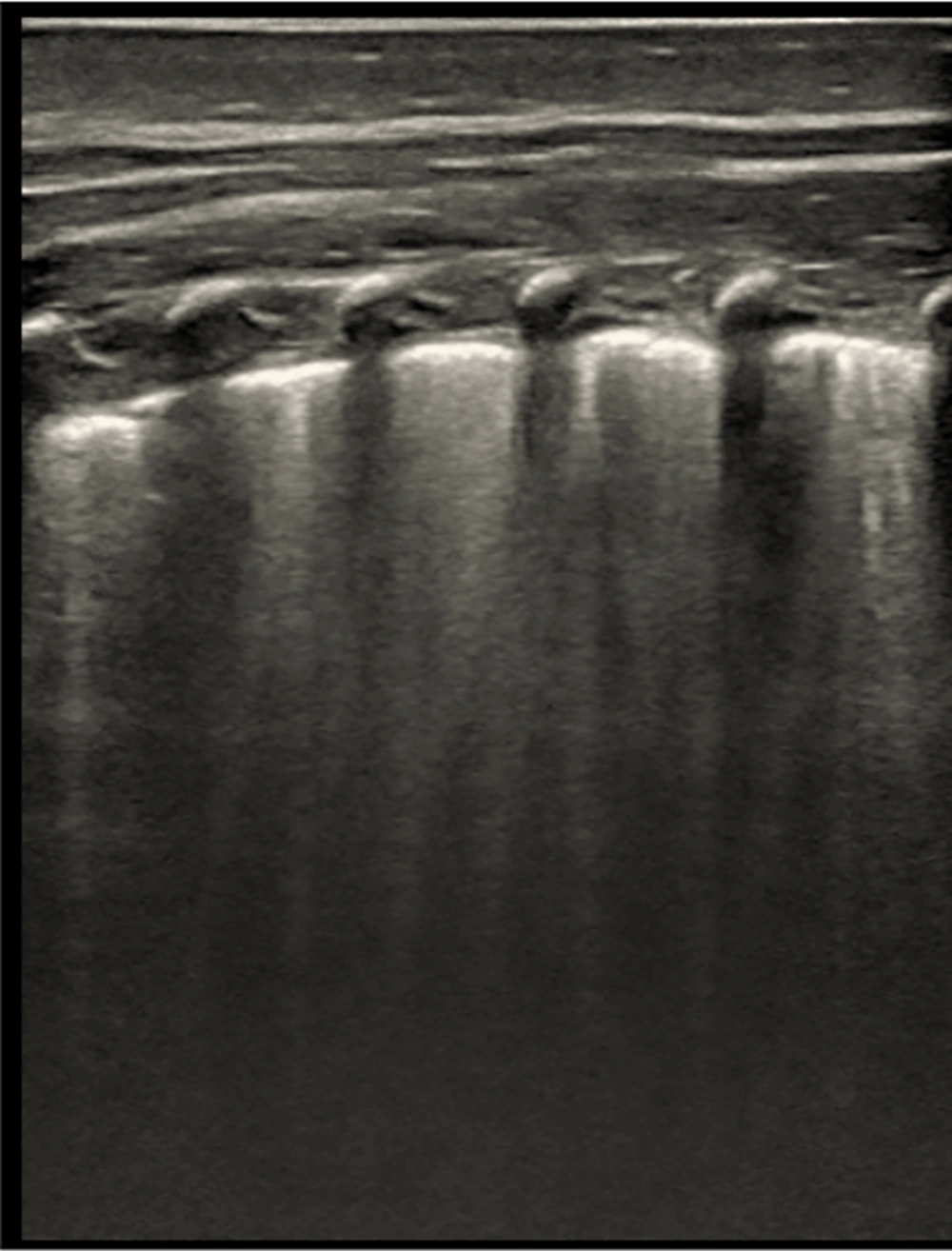
B-line artifacts in an ultrasound image. Multiple, merging B-lines, focal abnormalities of the pleural line, and no A-lines are visible in this picture. An increase in interstitial lung fluid is strongly suggested by this trend. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
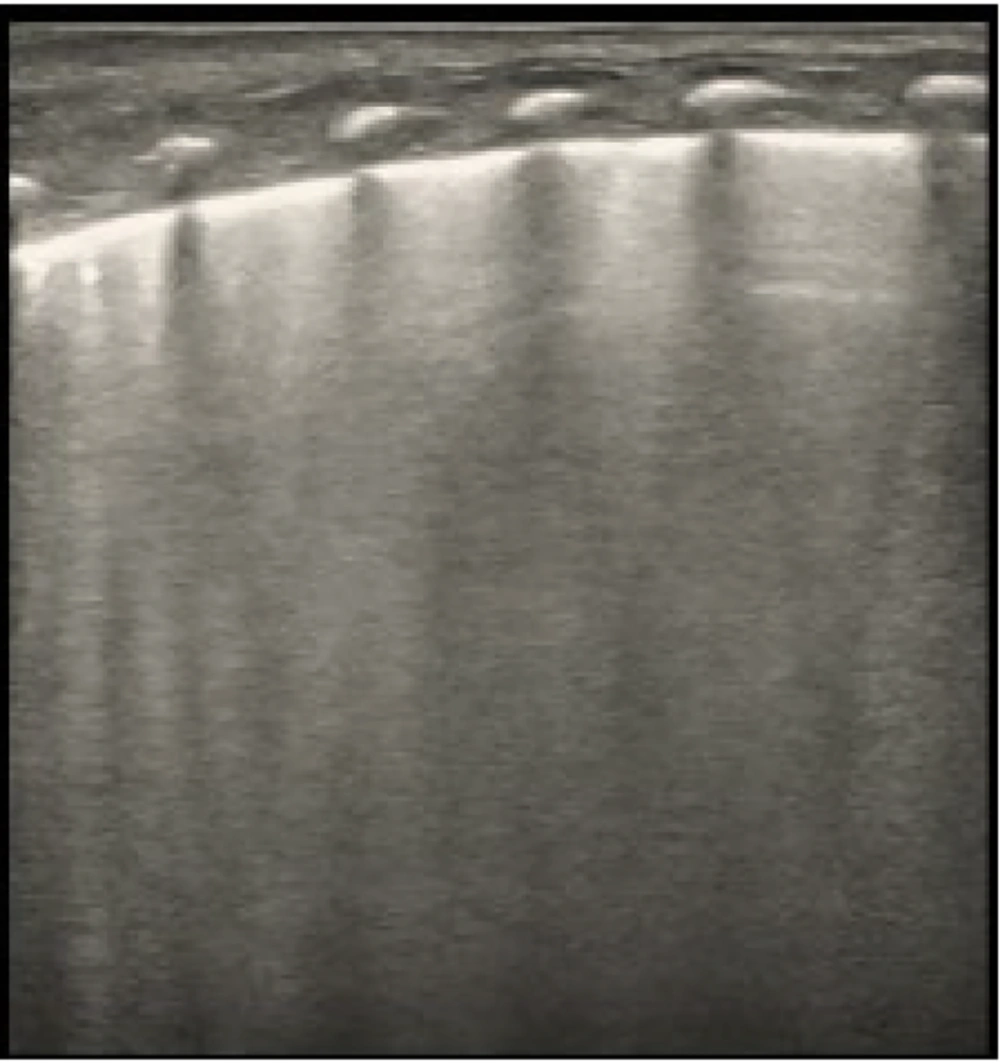
Alveolar-interstitial syndrome (AIS) is depicted in an ultrasound image. Findings consistent with AIS are seen in the scan, including localized thickening and blurring of the pleural line, total lack of A-lines, and lack of lung consolidation. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
Ultrasound image indicative of neonatal respiratory distress syndrome (NRDS). Large-scale lung consolidation with noticeable air bronchograms, no A-lines, and either thicker or absent pleural lines are all present in this image, which is consistent with NRDS. The NRDS can be diagnosed by combining clinical case characteristics. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
Acute respiratory distress syndrome (ARDS)-consistent ultrasound image. This picture shows signs of ARDS, including thickened or absent pleural lines, total lack of A-lines, and severe lung consolidation with conspicuous air bronchograms. The ARDS can be diagnosed by combining clinical case characteristics. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
Ultrasound picture showing pneumonia of newborn (PN). The picture shows PN-like lung consolidation with noticeable air bronchograms, no A-lines, and either thickened or absent pleural lines. Pneumonia can be diagnosed by combining clinical case characteristics. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
Ultrasound image suggestive of neonatal pulmonary hemorrhage (NPH). With fragmented tissue-like patterns, prominent air bronchograms, no A-lines, and either absent or thicker pleural lines — all of which are indicative of NPH — the image displays heterogeneous echogenicity. Pneumonia can be diagnosed by combining clinical case characteristics. A Mindray M9 portable color Doppler ultrasound machine (Shenzhen Mindray Biomedical Electronics Co., Ltd., China) was used to take the pictures.
4.3. Comparison of Ultrasonographic Results Between Neonates with Transient Tachypnea of Newborn and Healthy Controls
A comparison of ultrasonographic results between 88 neonates with TTN and 97 healthy controls is shown in Table 2. No pulmonary consolidation was found when ultrasound images of neonates without pulmonary illness and those with TTN were compared. Significant differences were observed between TTN patients and neonates without pulmonary disease in terms of white lung, dense B-lines, AIS, B-lines, comet tail sign, bipulmonary spots, pleural effusion, pleural line, and A-line (P < 0.05) (Table 2). Both TTN patients and newborns without pulmonary illness may have pleural effusion and the B-line.
4.4. Comparison of Frequency of B-lines in Neonates with Transient Tachypnea of Newborn Stratified by Gestational Age
According to Table 3, there were statistically significant differences (P < 0.05) between the TTN group and the control group when comparing the frequency of B-lines in newborns at ≤ 32 weeks, ≤ 34 weeks, and ≤ 37 weeks of gestational age. However, when comparing the TTN group to the control group at 42 weeks, there was no statistically significant difference (P > 0.05) in the emergence of B-lines.
Advice: B-lines are prevalent in neonates without pulmonary disease. TTN can occur in both full-term and premature infants, and the incidence of TTN steadily declines with increasing gestational age.
4.5. Duration of B-lines in Newborns with TTN Stratified by Gestational Age
Table 4 displays the duration of B-lines in newborns with TTN stratified by gestational age. All gestational age groups showed significant variations (P < 0.05). When comparing the TTN group to the controls, the duration of B-lines was substantially longer [77.09 hours (95% CI, 73.42 - 80.76) to 221.75 hours (95% CI, 212.80 - 230.70) vs. 48.12 hours (95% CI, 45.80 - 50.44) to 143.23 hours (95% CI, 137.27 - 149.19), P < 0.05)]. However, B-lines alone are not sufficient to diagnose TTN. TTN newborns, particularly those in the lower gestational age group, exhibited a substantially longer duration of B-lines compared to the control group, suggesting a longer duration of interstitial pulmonary fluid accumulation. This finding has clinical implications for forecasting the degree and duration of respiratory distress in TTN newborns and may inform clinical decision-making.
5. Discussion
Pneumothorax and atelectasis in neonates require prompt diagnosis and treatment, as do common lung conditions such as TTN, NRDS, ARDS, MAS, PN, NPH, and BPD (1, 3, 5-14). After examining the sonographic aspects of prevalent lung disorders, we found that some do not entirely match earlier studies, necessitating further research.
5.1. Discovery of Common Pulmonary Diseases in Newborns by Lung Ultrasound
Lung sliding and pleural lines are basic ultrasonographic characteristics essential for interpreting neonatal LUS images. Our results align with earlier research showing that pleural line anomalies, including obliteration, roughness, or irregularity, indicate underlying pathology (1-4). The acoustic impedance difference between the lung and pleura, pleural thickness, pleural cavity size, and the fluid and air content of the lung all affect pleural line thickness. Additionally, the appearance of the pleural line alone cannot be used as a sign of abnormalities because mechanical factors also play a role. Contrary to Liu’s findings, the number of A-lines cannot be used to determine the presence or absence of pathology alone because it is sensitive to changes in ultrasound parameters like power, focus, depth, and gain and is directly correlated with the fluid and air content within the lung (2, 4). By enabling sound to pass through the lung tissue, LUS examination is useful in identifying pleural-lung disorders. However, pneumothorax or subcutaneous emphysema makes it difficult to see deep-seated lung conditions in those areas. In other words, not every underlying pathology is necessarily ruled out by a normal LUS appearance. Additional imaging modalities, such as chest X-rays or CT scans, are necessary for an appropriate diagnosis if clinical observations differ from LUS results.
5.2. Lung Ultrasound Examination for Fluid and Pulmonary Edema
Increased fluid content in the lung is indicated by B-lines, which are linked to factors such as image depth settings and the gas-liquid ratio inside the lung. According to earlier research, normal newborns may occasionally show a few B-lines, which typically disappear 1 - 7 days after birth (2, 4). Our research indicates that the duration of B-lines is related to treatment outcomes, postnatal illness, and gestational age. Although TTN can occur in term newborns, our research shows that it is more common in neonates with lower gestational ages. The amount of pulmonary fluid and its clearance capacity determine whether TTN is present; children born at lower gestational ages usually have reduced clearance capacities and longer clearance times. Alveolar-interstitial syndrome, dense B-lines, "white lung" and prolonged persistence of B-line abnormalities on LUS are all signs of TTN’s elevated pulmonary fluid content. Although these are characteristics of pulmonary edema, identical LUS observations can also be seen in conditions other than TTN. Additionally, early in the postnatal period, B-lines can be seen in non-pulmonary illnesses. Therefore, these data alone cannot be used to diagnose TTN. Contrary to some earlier findings, our research also showed that the presence of pulmonary point signals is not a special feature of TTN but can occur in illnesses such as respiratory distress syndrome, PN, and meconium aspiration syndrome (2, 4, 5, 15).
5.3. Consolidation and Atelectasis Diagnosis by Lung Ultrasound
We combine NRDS, ARDS, MAS, PN, NPH, and BPD for analysis since our results show that these conditions are characterized by consolidation on LUS. These results are consistent with earlier research (16-18). Similar ultrasonographic findings to consolidation are seen in neonatal atelectasis, but there are no pleural effusions or pulmonary point symptoms, which differs from several earlier reports (2, 4, 6-13, 19-22). According to our experience, LUS by itself is not a good indicator of BPD. When oxygen reliance persists for more than 28 days and if LUS shows patchy consolidation and anomalies in the pleural line (apart from massive consolidation and other curable reasons), this method of diagnosing BPD might produce a more dependable and clinically sound result. Crucially, lung consolidation on LUS alone cannot be used to diagnose a particular lung disease; rather, it should result in a classification into a group of disorders, following which clinical presentation and other diagnostic information should be incorporated into the evaluation. The "patchy sign" indicates consolidation or hemorrhage in lung tissue, whereas the "snowflake sign" and "ground-glass opacity" are LUS findings that show lung consolidation and/or atelectasis. These results emphasize the importance of understanding the clinical correlations of these symptoms when using a diagnostic strategy.
5.4. Lung Ultrasound for Diagnosing Pneumothorax
Our results on pneumothorax are consistent with those of other investigations (2, 4). In the studied location, lung sliding (sandpaper or coast sign in M-mode) aids in ruling out pneumothorax. On M-mode ultrasonography, the air in the pleural space causes acoustic shadowing, which impairs lung sliding and results in a layering or barcode sign that denotes pneumothorax. However, when pneumothorax is present, deep-seated lung illness may not be apparent. Although the absence of pulmonary point symptoms does not rule out the potential of pneumothorax in severe cases, they can be useful in defining the boundaries of mild to moderate pneumothoraces. Assessment of pulmonary point signs, lung sliding, and B-lines using both B-mode and M-mode ultrasound should be part of a thorough evaluation when pneumothorax is suspected. To rule out pneumothorax, look for B-lines or lung sliding. Pleural effusion, pulmonary edema, consolidation, and pneumothorax can all be diagnosed with the benefits of LUS (2, 4, 23). By examining multiple infant lung conditions, this study circumvents the drawbacks of single-disease research. Drawbacks of LU include its incapacity to identify deep-seated lung disease in infants with pneumothorax and its incapacity to identify underlying pathology in the absence of anomalies at the pleural-lung interface. Additional investigation is required, including a correlation with the assessment of pulmonary edema by cardiac ultrasonography. In conclusion, screening and monitoring are made possible by the unique LUS patterns present in prevalent infant lung disorders. Lung ultrasound results must be carefully combined with the patient's clinical presentation and additional diagnostic information to make a diagnosis.
5.5. Innovation and Limitations of Research
This study is novel because it examined several prevalent lung conditions simultaneously to prevent inaccurate findings from focusing on only one. More objective ultrasonographic characteristics and alterations in prevalent infant lung diseases can be obtained using it. The diagnosis of pleural effusion, pulmonary edema, pulmonary consolidation, and pneumothorax is relatively straightforward with LUS, which is one of its numerous benefits (2, 4, 23). However, it should be acknowledged that the following are the limitations of LUS diagnosis: Lesions in the lung tissue may not be diagnosed when no lesions at the pleura-lung interface are found in LUS diagnostics. It is also impossible to diagnose lung abnormalities in the deep areas of a pneumothorax.