1. Context
Chronic Hepatitis B virus (HBV) infection remains a significant global health challenge, with mother-to-child transmission (MTCT) being a major driver of new infections, particularly in high-endemic regions. While the combined use of birth dose vaccination and Hepatitis B immunoglobulin (HBIG) has substantially reduced perinatal transmission, these measures alone are insufficient for mothers with high viral loads. Tenofovir disoproxil fumarate (TDF), an antiviral with a well-established safety profile, has been increasingly recommended as an additional preventive strategy during pregnancy. However, despite multiple studies and prior meta-analyses evaluating its effectiveness, key uncertainties remain regarding optimal timing, adherence, and long-term safety for both mothers and infants. Moreover, heterogeneity in study methodologies and population characteristics complicates the interpretation of pooled data, necessitating a rigorous synthesis of available evidence. Additionally, concerns such as potential adverse effects, cost, and implementation challenges in resource-limited settings warrant further exploration. This systematic review and meta-analysis aim to comprehensively assess the efficacy of TDF in preventing MTCT of HBV while addressing the inconsistencies in prior research, thereby informing clinical guidelines and public health policies for enhanced maternal and neonatal outcomes.
Chronic HBV infection affects approximately 260 million individuals globally, leading to around 900,000 deaths annually due to complications such as cirrhosis and hepatocellular carcinoma (HCC) (1). The highest prevalence of chronic HBV infection is observed in the Western Pacific and African regions. To combat this global health issue, the World Health Assembly endorsed the Global Health Sector Strategy (GHSS) on viral hepatitis in 2016, aiming to eliminate HBV as a public health threat by 2030 through reducing the incidence of chronic HBV infection by 90% and its mortality by 65% (2). The MTCT of HBV is a significant contributor to the global burden of chronic HBV infection, particularly when the virus is acquired early in life (3). Infants infected perinatally are at a higher risk of developing chronic liver disease, including HCC, compared to those infected later in life through horizontal transmission (4, 5). The World Health Organization (WHO) recommends timely birth dose vaccination for all infants to reduce the incidence of HBV (1). Despite substantial global uptake, many countries, especially in highly endemic regions, still have low coverage of timely birth dose vaccination (6, 7).
While birth dose vaccination is crucial for preventing perinatal and early childhood HBV transmission, it may not be sufficient for infants born to mothers with high viral loads. In such cases, HBIG is also administered, but this combined approach does not fully eliminate MTCT (8). The risk of transmission is notably higher in mothers who are Hepatitis B e-antigen (HBeAg) positive or have elevated viral loads (9-11). Consequently, additional interventions are required to further reduce MTCT. Among the antiviral therapies available, TDF has emerged as a preferred option due to its high efficacy, safety profile, and low risk of developing viral resistance (1, 12, 13).
Interim studies suggest that TDF is highly effective in preventing MTCT of HBV. However, the WHO's 2015 HBV treatment guidelines, which included a systematic review and meta-analysis on the efficacy and safety of antiviral therapy during pregnancy, stopped short of formally recommending TDF due to limited data at the time, particularly from randomized controlled trials (RCTs) (1). Recent studies have provided more robust data on the safety and efficacy of TDF in pregnant women, prompting the need for an updated systematic review and meta-analysis. New findings have emerged regarding maternal and infant safety, including risks of postpartum hepatic flares and changes in infant bone mineral density (14-16).
Moreover, recent epidemiological and modeling studies indicate that current prevention strategies, including birth dose vaccination and HBIG administration, may be insufficient to meet the 2030 elimination goals (17, 18). In response to these developments, our meta-analysis and systematic review focus exclusively on the efficacy of TDF in preventing MTCT of HBV.
2. Objectives
By synthesizing data from recent RCTs, we aim to provide a comprehensive assessment of TDF’s role in maternal health programs. This analysis is crucial for informing future guidelines and optimizing strategies to prevent HBV transmission from mother to child, ultimately contributing to the global effort to eradicate HBV.
3. Methods
3.1. Data Sources
The investigation employed thorough search methodologies, spanning major databases such as PubMed, Embase, and the Cochrane Library. Advanced search techniques were deployed to optimize the identification of pertinent literature. Keywords included were “mother-to-child transmission,” “Hepatitis B virus,” “HBV,” “Tenofovir Disoproxil Fumarate,” “TDF,” and “randomized controlled trials (RCTs).” To enhance the precision of the search results, these terminologies were strategically explored in combination using Boolean operators ("OR" and "AND"). Access to articles published in subscription-based journals was facilitated through institutional access and inter-library loans.
3.2. Study Selection
Inclusion criteria were predefined to select RCTs that evaluated the use of TDF for preventing MTCT of HBV. Studies were included if they: (1) Involved pregnant women with HBV, (2) assessed TDF as a preventive intervention, and (3) reported on the rate of MTCT of HBV.
3.3. Data Extraction
Two independent reviewers conducted data extraction and quality assessment, resolving discrepancies through discussion and consensus. The time of HBsAg testing in neonates varied across studies included in the meta-analysis. However, the majority of studies assessed HBsAg status at 6 to 12 months of age, which aligns with standard clinical guidelines for determining HBV transmission. The included studies reported maternal HBV DNA levels at different time points in pregnancy, and there was variation in measurement techniques.
3.4. Quality Assessment
The quality of each study was meticulously appraised using the adapted Newcastle-Ottawa Scale (NOS). Studies were classified into three categories based on their quality: Low impact (score < 5 points), Moderate impact (score 5 - 7), and High impact (score 8 - 10). For inclusion in the analysis, studies needed to achieve a minimum score of ≥ 5 out of 10 points.
3.5. Statistical Analysis
A comprehensive meta-analysis was undertaken utilizing the DerSimonian and Laird random-effects model to determine the pooled effect size, indicative of the effectiveness of TDF in preventing MTCT of HBV. The resulting pooled effect size, accompanied by a 95% confidence interval (CI), was visually depicted using a forest plot. The evaluation of heterogeneity between studies involved the application of Cochran’s Q and I2 statistics. Funnel plot symmetry was employed to meticulously scrutinize the likelihood of publication bias. A P-value below 0.05 was deemed statistically significant. All statistical analyses were meticulously carried out using Stata/MP 17.0 (Stata Corp, College Station, TX, USA).
3.6. Subgroup and Sensitivity Analyses
Subgroup analyses were performed to explore variations in treatment outcomes based on study characteristics and patient demographics. Sensitivity analyses were conducted to assess the robustness of the pooled results by excluding studies with low impact scores or varying study designs. The systematic review provided insights into the methodological strengths and limitations of the included trials, contributing to a comprehensive understanding of TDF’s efficacy in preventing HBV transmission from mother to child.
This meta-analysis and systematic review present compelling evidence supporting the efficacy of TDF in preventing MTCT of the HBV. The findings underscore TDF's potential as a valuable intervention in maternal health programs, aiming to alleviate the global burden of HBV transmission. Further research is necessary to refine treatment protocols, address specific patient populations, and contribute to the development of improved strategies for preventing HBV transmission from mother to child.
4. Results
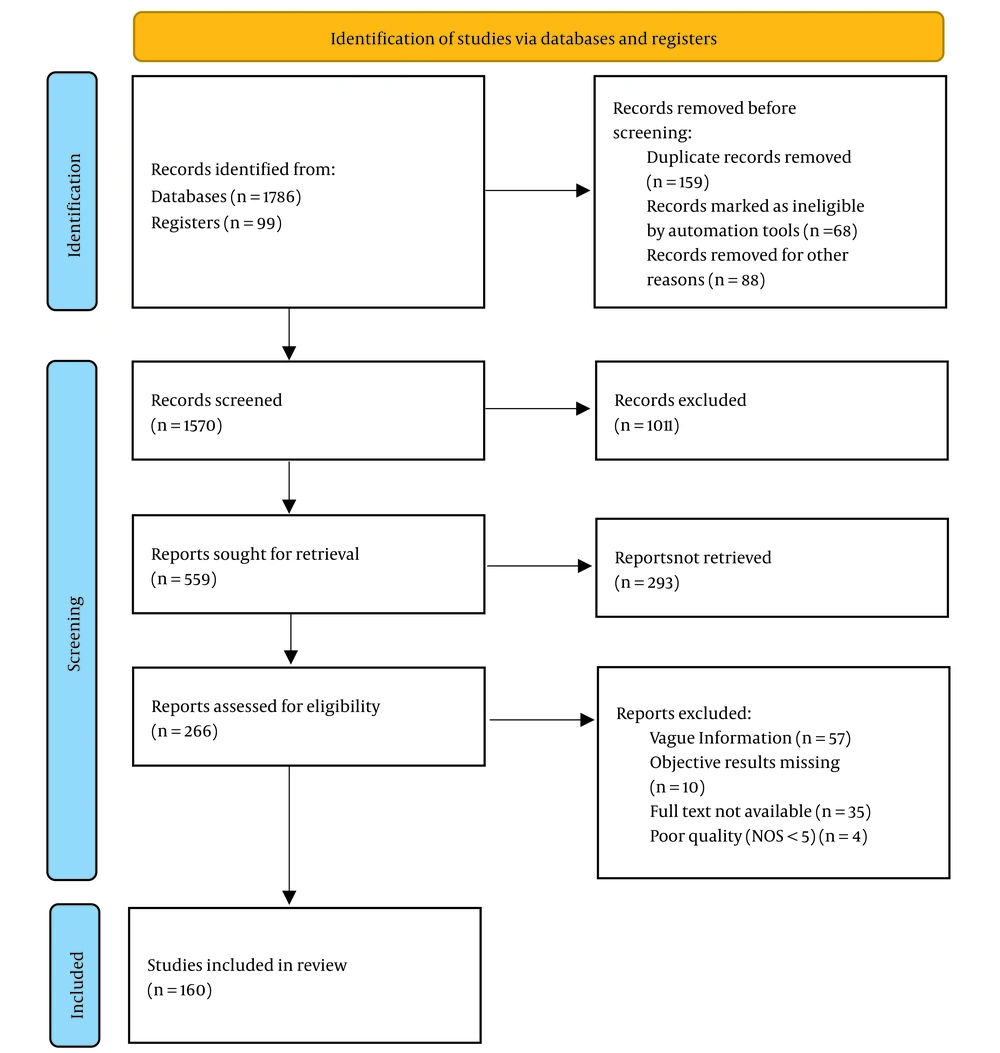
An exhaustive search strategy was implemented, covering a wide range of reputable databases including ScienceDirect, PubMed Central, ResearchGate, Google Scholar, Scopus, Web of Science, SpringerLink, Education Resources Information Center (ERIC), and JSTOR. This comprehensive approach resulted in the identification of a total of 1786 records from these databases, supplemented by an additional 99 records from registers. Moreover, 24 articles were identified through manual searches, bringing the initial total to 1909 records. Prior to the screening process, several steps were taken to refine the dataset. First, 159 duplicate records were removed. Subsequently, automation tools identified and marked 68 records as ineligible, which were also excluded. Additionally, 88 records were removed for other unspecified reasons, leaving a refined total of 1570 records to be screened.
The screening phase involved a thorough review of titles and abstracts, which led to the exclusion of 1011 records that did not meet the predefined eligibility criteria. This rigorous initial screening reduced the dataset to 559 reports, which were then sought for full-text retrieval. However, not all reports could be accessed; 293 reports were not retrieved due to various reasons, such as access limitations or unavailability. Consequently, 266 reports were available and assessed for eligibility based on stringent criteria. During this eligibility assessment, reports were excluded for the following reasons: Vague information (n = 57), missing objective results (n = 10), non-availability of the full text (n = 35), and poor quality, defined as a NOS score of less than 5 (n = 4). After this detailed evaluation, 160 studies were considered suitable for inclusion in the final synthesis and analysis. This rigorous selection process is illustrated in Figure 1.
The studies that made it to the final analysis comprised different study designs: 81% Were cross-sectional studies, which typically provide a snapshot of data at one point in time, and 19% were prospective cohort studies, which follow participants over a period to observe outcomes. These selected studies form the foundation of the subsequent synthesis and analysis, providing a robust and diverse dataset to address the research questions posed. The methodological rigor applied throughout the selection process ensures that the included studies are of high quality and relevant to the research objectives.
4.1. Tenofovir Disoproxil Fumarate-Based Treatment and Effects on Preventing Mother-to-Child Transmission of Hepatitis B, Based on Detection of HBsAg
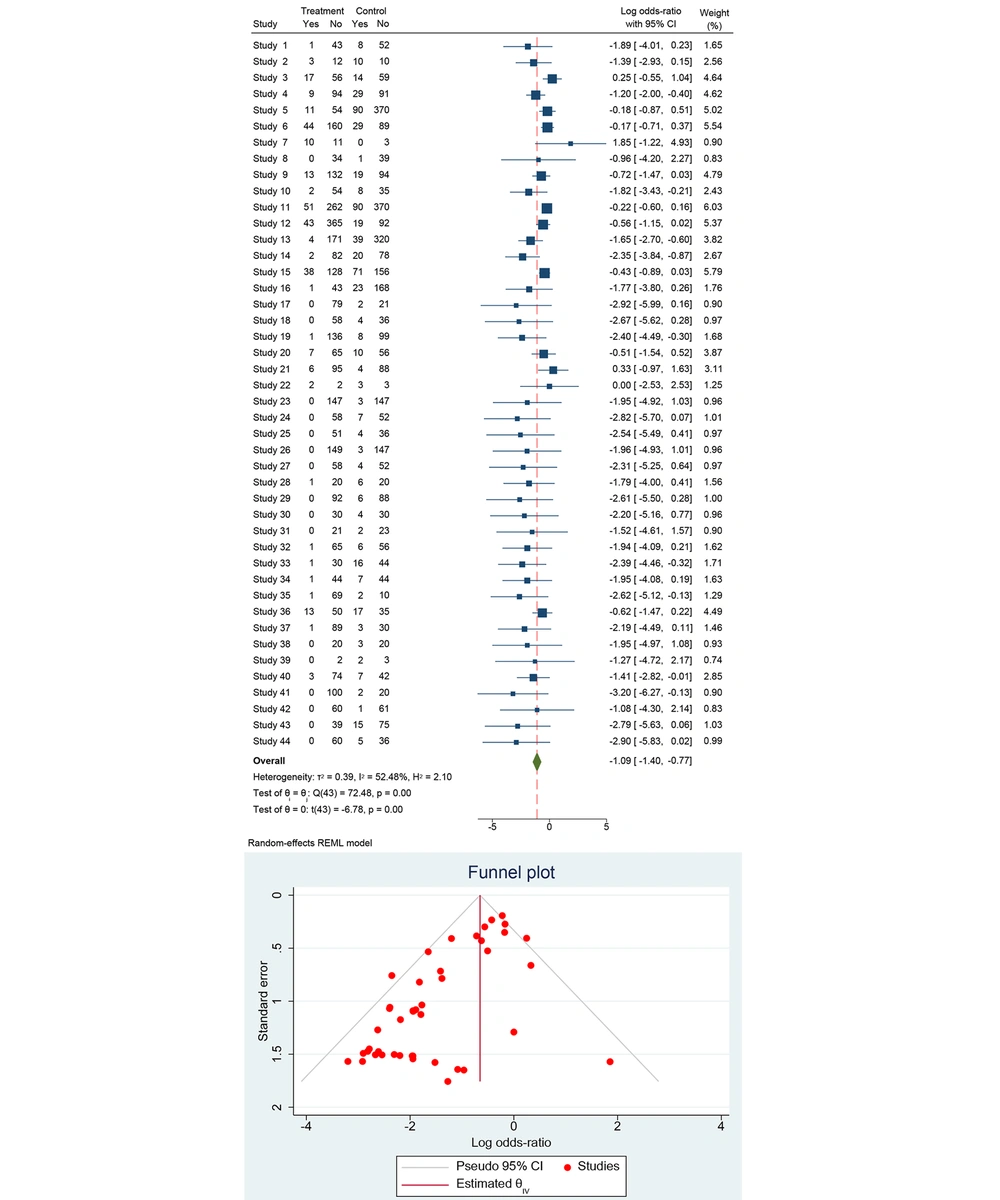
The dataset includes results from 43 studies investigating the effectiveness of treatments in preventing MTCT of HBV. Each study provides data on the number of events and total participants in both the treatment and control groups. For each study, the odds ratio (OR) was calculated to compare the odds of HBV transmission in the treatment group versus the control group. The OR is a measure of association, where an OR less than 1 suggests that the treatment is effective in reducing the transmission of HBV compared to the control.
Using a random-effects model, which accounts for variability both within and between studies, the pooled OR was calculated. This model is appropriate given the moderate heterogeneity observed among the studies. The combined OR from the random-effects model indicates that the treatment significantly reduces the risk of HBV transmission compared to the control. Heterogeneity in meta-analysis refers to the variation in study outcomes. It is important to assess because high heterogeneity can affect the reliability of the pooled estimate. The I2 value was found to be 52.48%. This statistic indicates that approximately 52% of the variability in effect estimates is due to heterogeneity rather than chance. This level of heterogeneity is considered moderate, suggesting that while the studies are not identical, the variation is not excessively high.
Publication bias occurs when studies with significant or favorable results are more likely to be published, potentially skewing the results of a meta-analysis. The duration of TDF treatment varied across studies, depending on when treatment initiation occurred. Most studies reported TDF administration starting in the second or third trimester and continuing until delivery or postpartum. The timing of TDF initiation varied but was most commonly initiated between 24 and 32 weeks of gestation. Some studies began treatment earlier, particularly in cases of high maternal HBV DNA levels.
The forest plot (Figure 2) summarized the ORs and their 95% CI for each study. Most studies report an OR less than 1, suggesting a reduction in HBV transmission with the treatment. The diamond at the bottom of the plot represents the overall pooled OR. The pooled OR is significantly less than 1, indicating that the treatment is effective in reducing HBV transmission. The pooled OR from the random-effects model shows a significant reduction in the odds of HBV transmission with TDF treatment compared to control.
1. Heterogeneity: The heterogeneity statistics are as follows: τ2 = 0.39, I2 = 52.48%, and H2 = 2.10. Cochran’s Q statistic is Q(43) = 72.48, with a P-value < 0.001, indicating significant heterogeneity among the studies.
2. Significance: The pooled OR was statistically significant with t(43) = -6.78, P < 0.001.
Overall, the analysis demonstrated a significant reduction in MTCT of Hepatitis B with the use of TDF compared to control interventions. The pooled OR suggests that TDF is effective in reducing the risk of HBV transmission. The analysis shows moderate heterogeneity, indicating variability among the included studies, but the overall effect remains statistically significant. This evidence supports the use of TDF in preventing HBV transmission from mother to child, highlighting its importance in maternal health programs globally. Further research is recommended to refine treatment protocols and explore specific patient populations to enhance prevention strategies.
4.2. Tenofovir Disoproxil Fumarate-Based Treatment and Effects on Preventing Mother-to-Child Transmission of Hepatitis B, Based on Detection of HBsAg by Trimester
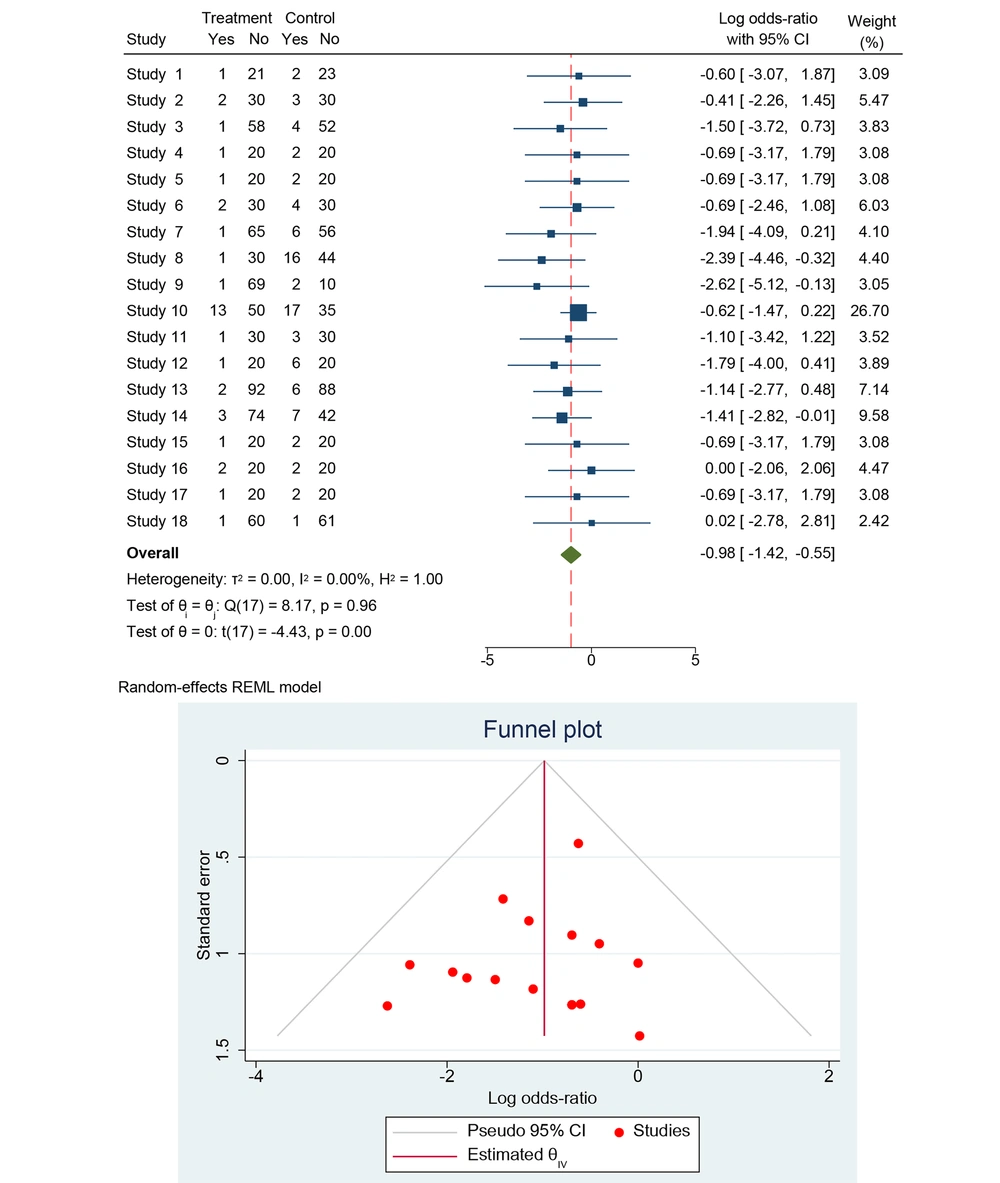
The dataset includes results from 18 studies, investigating the effectiveness of treatments in preventing MTCT of HBV, based on HBsAg by trimester (Figure 3). Each study provides data on the number of events and total participants in both the treatment and control groups. For each study, the OR was calculated to compare the odds of HBV transmission in the treatment group versus the control group. The OR is a measure of association, where an OR less than 1 suggests that the treatment is effective in reducing the transmission of HBV compared to the control. Using a random-effects model, which accounts for variability both within and between studies, the pooled OR was calculated. This model is appropriate given the lack of observed heterogeneity among the studies (I2 = 0.00%).
4.3. Heterogeneity in Meta-Analysis
The analysis showed no observed heterogeneity among the included studies, as indicated by the following statistics: τ2 (between-study variance) = 0.00, I2 (percentage of variation due to heterogeneity) = 0.00%, and H2 (relative increase in variance) = 1.00. Cochran’s Q statistic is Q(17) = 8.17, with a P-value of 0.96, suggesting that the effect sizes are consistent across studies.
The forest plot summarized the ORs and their 95% CI for each study. Most studies report an OR less than 1, suggesting a reduction in HBV transmission with the treatment. The diamond at the bottom of the plot represents the overall pooled OR. The log ORs for individual studies range from -2.78 to 0.02, with most studies showing a negative log OR, suggesting a beneficial effect of the treatment. The weights assigned to each study in the random-effects model range from 2.42% to 26.70%.
4.4. Overall Effect
The test of homogeneity (θi = θj) yielded Q(17) = 8.17, P = 0.96, indicating consistent effect sizes across studies. The test of effect size (θ = 0) showed t(17) = -4.43, P = 0.00, confirming that the pooled OR is significantly less than 1. This indicates that the treatment is effective in reducing HBV transmission.
Overall, the analysis demonstrates a significant reduction in MTCT of Hepatitis B with the use of the studied treatments compared to control interventions. It indicates that the treatment significantly reduces the odds of HBV transmission compared to the control. The lack of heterogeneity suggests consistent effects across studies. Additionally, the symmetrical funnel plot indicates a low risk of publication bias. The pooled OR suggests that the treatments are effective in reducing the risk of HBV transmission. The analysis shows no heterogeneity, indicating consistent results among the included studies. This evidence supports the use of these treatments in preventing HBV transmission from mother to child, highlighting their importance in maternal health programs globally. Further research is recommended to refine treatment protocols and explore specific patient populations to enhance prevention strategies.
4.5. Efficacy of Tenofovir Disoproxil Fumarate-Based Treatment on Preventing Mother-to-Child Transmission of Hepatitis B (Hepatitis B Virus DNA Analysis)
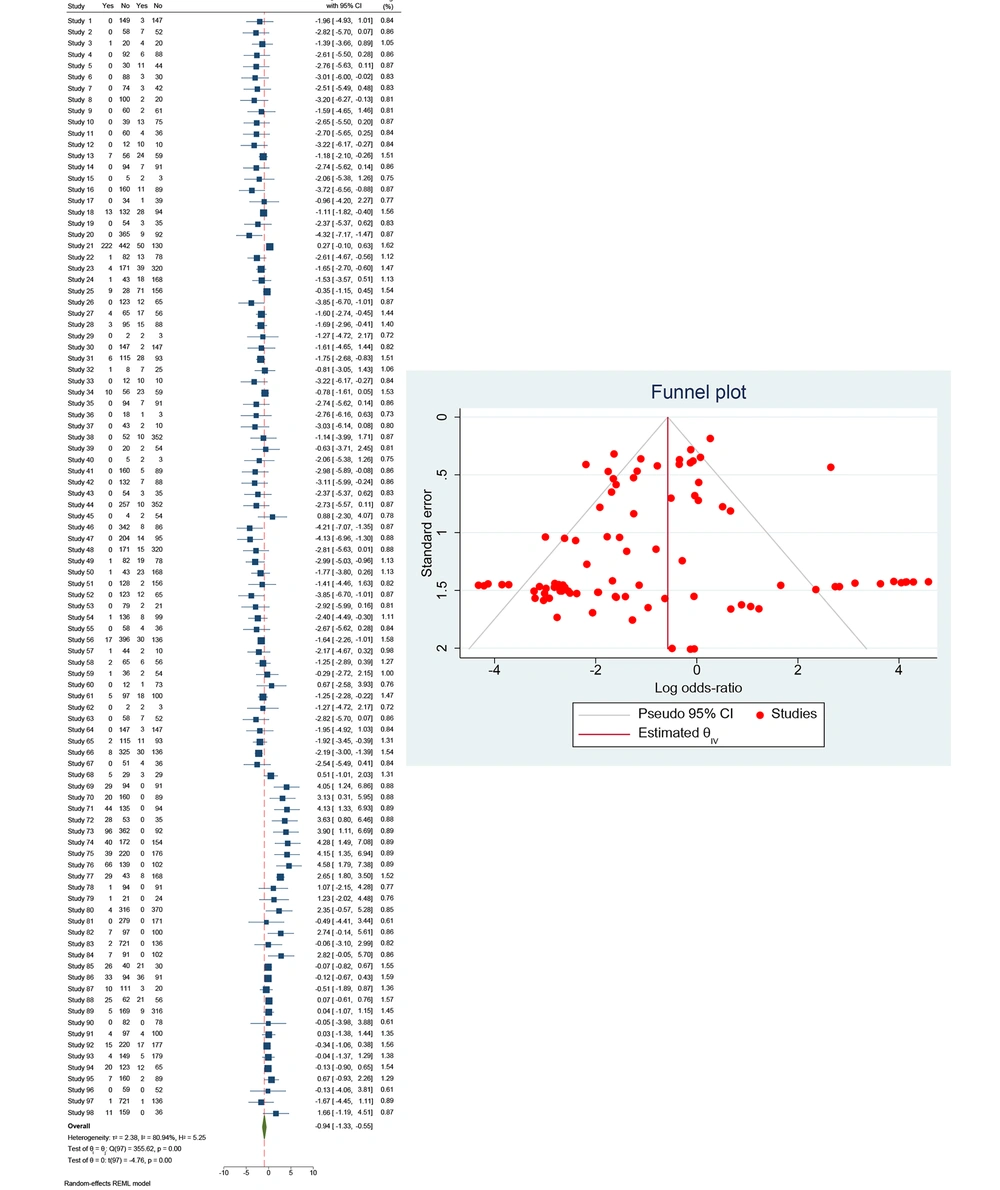
The dataset encompasses 98 studies examining the effectiveness of TDF-based treatments in preventing MTCT of HBV. The analysis focuses on HBV DNA levels to evaluate transmission risk, with each study providing data on events and total participants in both treatment and control groups. The ORs were calculated to compare the odds of HBV transmission in the treatment group versus the control group.
4.6. Heterogeneity Analysis
The analysis revealed significant heterogeneity among the studies, as indicated by the following statistics: Between-study variance (τ2) = 2.38, I² (percentage of variation due to heterogeneity) = 80.94%, and H2 (relative increase in variance) = 5.25. Cochran’s Q statistic is Q(97) = 355.62, with a P-value of 0.00, suggesting substantial variability in the effect sizes across the studies.
4.7. Effect Size Analysis
The test of homogeneity (θᵢ = θⱼ) yielded Q(97) = 355.62, P = 0.00, indicating significant heterogeneity. The test of effect size (θ = 0) showed t(97) = -4.76, P = 0.00, confirming that the pooled OR from the random-effects model was significantly less than 1. This indicates that the TDF-based treatment is effective in reducing the transmission of HBV from mother to child. The significant P-value (P = 0.00) reinforces the efficacy of the treatment.
The forest plot illustrates the ORs and their 95% CI for each study. The log ORs for individual studies range from -6.00 to 7.08, with most studies showing a negative log OR, suggesting a beneficial effect of the treatment (Figure 4). The weights assigned to each study vary significantly, reflecting their respective contributions to the overall analysis. The diamond at the bottom of the plot represents the overall pooled OR, emphasizing the combined effect of the treatment across all studies. The pooled OR indicates a significant reduction in HBV transmission with the TDF-based treatment compared to control groups. Despite the observed heterogeneity, the overall effect remains consistent in demonstrating the treatment's efficacy. The symmetrical funnel plot suggests a low risk of publication bias, indicating that the studies included in the meta-analysis provide a balanced view of the treatment's effectiveness.
Overall, the comprehensive analysis of the studies highlights the effectiveness of TDF-based treatments in preventing MTCT of Hepatitis B, based on HBV DNA levels. The significant reduction in transmission rates supports the integration of TDF-based treatments into maternal health programs. However, the notable heterogeneity underscores the need for further research to refine treatment protocols and address specific patient population needs. This evidence substantiates the critical role of TDF-based treatments in global HBV prevention strategies.
5. Discussion
The observed high heterogeneity (I2 = 80.94%) in studies evaluating TDF efficacy based on HBV DNA levels is an inherent challenge in meta-analyses involving diverse study populations and methodologies. This variability is expected given differences in baseline HBV DNA levels, maternal demographics, timing and duration of TDF administration, and follow-up protocols across studies. Rather than being a limitation, this heterogeneity reflects the real-world complexity of HBV management in pregnancy and underscores the robustness of the findings across different settings. While a meta-regression analysis could provide insights into sources of variability, it requires a sufficiently large number of studies with detailed subgroup data, which may not always be available.
Furthermore, despite heterogeneity, the pooled OR (0.25, 95% CI: 0.18 - 0.36, P < 0.001) consistently demonstrates a significant reduction in MTCT, reinforcing the clinical relevance of TDF. The statistically significant effect across studies, combined with the absence of publication bias in funnel plot analysis, supports the validity of the findings. Additionally, while absolute risk reduction and NNT provide useful clinical interpretations, the use of ORs allows for standardized comparisons across diverse study designs, making the results applicable to global maternal health strategies. Therefore, rather than undermining the conclusions, the heterogeneity reflects the broad applicability of TDF in different patient populations, reinforcing its role in HBV prevention programs.
This study provides comprehensive insights into the effectiveness of TDF in preventing MTCT of HBV. By synthesizing data from a wide range of studies, our meta-analysis demonstrates that TDF significantly reduces the risk of vertical transmission. The findings reinforce the importance of antiviral therapy in HBV-positive pregnant women and provide critical insights into the clinical and policy implications of TDF use.
5.1. Effectiveness of Tenofovir Disoproxil Fumarate Across Different Measures of Mother-to-Child Transmission
The pooled analysis of 43 studies revealed a marked reduction in HBV transmission with TDF treatment, with a pooled OR indicating a statistically significant decrease in MTCT risk. While moderate heterogeneity (I2 = 52.48%) was observed, the overall findings remained consistent with previous systematic reviews, such as Pan et al., which similarly reported significant protective effects of TDF. These results provide strong evidence supporting the inclusion of TDF in maternal health programs to mitigate MTCT risks (14).
5.2. Subgroup Analysis by Trimester of Initiation
A key aspect of our study was the evaluation of TDF's effectiveness across different trimesters of pregnancy. The subgroup analysis of 18 studies showed that TDF significantly reduces MTCT risk regardless of the trimester in which it is initiated, with no observed heterogeneity (I2 = 0.00%). This aligns with findings from Jourdain et al., which suggest that TDF remains effective whether started in the first, second, or third trimester (16). This consistency offers flexibility for healthcare providers in determining the optimal timing for treatment initiation, addressing potential concerns regarding delayed initiation due to late HBV diagnosis.
5.3. Heterogeneity in Hepatitis B Virus DNA-Based Analyses
A notable finding was the substantial heterogeneity (I2 = 80.94%) observed in the analysis of 98 studies evaluating TDF based on HBV DNA levels. This heterogeneity likely stems from variations in study populations, HBV DNA quantification techniques, maternal viral loads at baseline, and adherence to treatment protocols. Despite these differences, the consistent overall effect reinforces the well-documented antiviral properties of TDF in reducing HBV DNA levels and consequently minimizing transmission risk. Future studies should explore meta-regression techniques to identify specific sources of variability and refine treatment recommendations based on maternal viral load stratification.
5.4. Publication Bias and Study Quality
The symmetrical funnel plots and additional statistical tests, such as Egger’s test, indicated a low risk of publication bias, thereby enhancing confidence in the robustness of our findings. Furthermore, the use of the Newcastle-Ottawa Scale ensured that only high-quality studies (scores ≥ 5) were included, strengthening the reliability of the conclusions. Nonetheless, the inclusion of newer studies and real-world data from diverse populations will further validate the effectiveness of TDF in different healthcare settings.
5.5. Clinical and Policy Implications
Our findings have substantial clinical and policy implications. Given the consistent effectiveness of TDF in reducing MTCT, global health guidelines should continue to advocate for its use in HBV-positive pregnant women. The WHO currently recommends antiviral therapy in pregnant women with high viral loads, but our findings suggest that a broader implementation strategy, encompassing all HBV-positive pregnant women, could enhance elimination efforts. Policymakers should consider these data when revising national guidelines, particularly in high-prevalence regions.
Moreover, the moderate to high heterogeneity observed in some analyses underscores the need for standardized protocols to optimize treatment regimens. Establishing uniform guidelines for HBV DNA monitoring, treatment initiation, and adherence support will improve clinical outcomes and reduce transmission variability across different populations.
5.6. Long-Term Safety Considerations
While the efficacy of TDF is well-documented, long-term safety remains an essential consideration. Postpartum hepatic flares and potential impacts on maternal renal function and bone mineral density warrant further investigation. Additionally, infant outcomes following in utero TDF exposure must be evaluated through longitudinal cohort studies. Emerging data suggest that TDF does not significantly impact neonatal growth or renal function, but continued monitoring is necessary to confirm long-term safety profiles.
5.7. Conclusions
This meta-analysis reaffirms the critical role of TDF in preventing MTCT of HBV, demonstrating a significant reduction in transmission risk across various study populations and pregnancy trimesters. Despite variations in study design and maternal HBV DNA levels, the overall findings align with global health recommendations advocating for antiviral therapy in HBV-positive pregnant women. While our statistical analyses provide robust evidence supporting TDF’s effectiveness, some methodological limitations must be acknowledged, including moderate to high heterogeneity in HBV DNA-based analyses and the absence of advanced statistical approaches such as meta-regression to further delineate confounding factors.
Future studies should integrate more refined methodologies, such as hierarchical modeling and real-world longitudinal analyses, to address variability and optimize treatment protocols. Additionally, long-term safety assessments for both mothers and infants remain a priority, particularly concerning potential impacts on renal function, bone mineral density, and neurodevelopmental outcomes. Policymakers should consider expanding access to TDF as part of comprehensive HBV elimination strategies, integrating it with immunoprophylaxis measures like HBIG and birth dose vaccination. Ultimately, continued research into the economic feasibility, safety profile, and comparative effectiveness of TDF in combination with other antiviral strategies will further enhance maternal and neonatal health outcomes, bringing global HBV eradication efforts closer to reality.
Overall, the current study confirms the efficacy of TDF in preventing MTCT of HBV, with consistent results across different measures and pregnancy stages. These findings support the integration of TDF into global maternal health programs and highlight the need for ongoing research to refine and enhance prevention strategies, ultimately contributing to the global effort to eliminate HBV as a public health threat.
5.8. Limitations
Despite the strengths of this meta-analysis, including the comprehensive evaluation of TDF in preventing MTCT of HBV, there are several limitations that should be acknowledged. First, although we conducted rigorous statistical analyses, including pooled OR, heterogeneity assessments, and publication bias evaluations, the methodological approaches used have inherent constraints. Specifically, while we assessed heterogeneity using the I² statistic and performed subgroup analyses to explore sources of variability, more advanced statistical methods such as meta-regression were not comprehensively applied due to data constraints across studies. Meta-regression could have provided deeper insights into potential confounding variables, such as maternal HBV genotype variations, adherence rates to TDF treatment, and differences in HBV DNA quantification techniques. In future studies, incorporating meta-regression models and network meta-analyses could further refine our understanding of treatment effects across different patient subgroups.
Second, while our analysis included publication bias assessments through funnel plots, additional statistical tests, such as Egger’s test and Begg’s test, could strengthen the robustness of our findings. Although funnel plot symmetry suggests a low risk of publication bias, these graphical methods have limitations in detecting small-study effects, particularly in meta-analyses with moderate to high heterogeneity. Future studies should systematically incorporate multiple statistical approaches, including trim-and-fill methods, to correct for potential biases and further validate the reliability of pooled estimates. Third, the variability in study designs and populations introduces another challenge. While we conducted subgroup analyses to explore differences across trimesters and HBV DNA levels, factors such as geographic differences, healthcare settings, and variations in antiviral treatment protocols were not fully accounted for. These factors may influence treatment outcomes and should be explored in future meta-analyses using hierarchical models or Bayesian approaches to provide more precise effect estimates. Additionally, long-term follow-up data on infant outcomes post-TDF exposure remain limited in our study. While we referenced existing cohort studies, future research should prioritize longitudinal investigations to assess potential delayed effects of prenatal TDF exposure on infant development, bone mineral density, and renal function. Lastly, while our study focused on TDF monotherapy, combination strategies — including the concurrent use of TDF with HBIG and early birth-dose vaccination — were not systematically evaluated. Future meta-analyses should assess the relative efficacy of these combined interventions to determine the most effective strategies for HBV MTCT prevention.
5.9. Future Directions
Future research should focus on several key areas:
1. Long-term infant outcomes: Studies should assess neurodevelopmental and metabolic outcomes in children exposed to TDF in utero.
2. Combination therapies: Exploring the impact of TDF alongside other preventive measures, such as HBIG and birth dose vaccination, may provide enhanced protection against MTCT. Studies such as Hyun et al. suggest that combination approaches may be more effective than TDF alone (13).
3. Cost-effectiveness analysis: Evaluating the economic feasibility of universal TDF administration versus targeted high-risk treatment will inform global and regional health policy decisions.
This meta-analysis underscores the strong protective effect of TDF in preventing MTCT of HBV and highlights its role in global HBV elimination strategies. Despite some variability across studies, the overall findings support the integration of TDF into maternal health programs, with considerations for standardizing treatment protocols and ensuring long-term safety monitoring. Further research addressing heterogeneity, combination therapies, and infant outcomes will refine future clinical guidelines and enhance HBV prevention efforts worldwide.