1. Background
Selenium (Se) is an essential element vital for human life. It contributes to the formation of many enzymes as a cofactor and plays a major role in various biological pathways such as thyroid hormone mechanisms, antioxidant enzyme defense, and immune system regulation. It is thought that the antioxidant properties of Se may be useful to prevent increased oxidative stress in autoimmune thyroid diseases (AITDs) (1-3). Proteins carrying Se in their active zones are defined as selenoproteins and are dependent on Se to function properly. The human body possesses approximately 100 selenoproteins, and 30 of them have been defined (4). The most prominent of these are the enzymes glutathione peroxidase (Gpx), thioredoxin reductase (TRS), and iodothyronine deiodinase (ID). Many selenoproteins contribute to specific biochemical reactions other than antioxidant defense, such as iodothyronine deiodinase (DIOs), which has a major role in thyroid hormone synthesis, glutathione peroxidase-4 (Gpx4), which contributes to spermatogenesis, and selenophosphate synthetase 2 (SPS2), which plays a part in selenoprotein synthesis. Selenoprotein P (SelP) and glutathione peroxidase-3 (Gpx3) activity can also be used as an indicator for plasma Se levels (5, 6).
Thyroid tissue contains high amounts of Se. Selenoproteins are important in thyroid enzymes and metabolism (7). Iodothyronine deiodinase-1, which carries Se, has been shown as the primarily responsible enzyme for peripheral T4-T3 transformation (5). Thyroid gland cells are protected from the oxidative stress of H2O2, which is essential for iodination during hormone synthesis, by the antioxidant effects of the selenoprotein-carrying enzyme Gpx (8). Selenium deficiency has been shown to cause a decrease in T4-T3 transformation, thus leading to an increased T4/T3 ratio in tissues with ID-1 and ID-2 (6, 8). Selenium deficiency prevents thyroid peroxidase (TPO) and Gpx from functioning normally, causing inefficient protection against free radicals, cell injury, and the eventual autoimmune destruction of the gland itself. Selenium replacement therapy may improve the level of inflammation for patients suffering from autoimmune thyroiditis (9, 10).
Selenium deficiency is not common among children and adolescents who consume a regular diet. Clinical findings such as darkened hair color, white nails, and cardiac dysfunction can be signs of Se deficiency (4, 11). While Se toxicity (selenosis) may be acute or chronic, it is not a common medical presentation among humans. Selenosis symptoms may include nausea, emesis, abdominal pain, diarrhea, brittle nails, and peripheral neuropathy (8, 11). Selenium can be found in food obtained from animal or plant sources. Dietary Se is primarily selenomethionine (11, 12). Up to half of Se is lost when food is cooked; however, this can be reduced by cooking at low pH. Scientific data recommends Se replacement therapy for pediatric patients whose Se levels are below 4 g/dL. The minimum uptake required to prevent deficiency-related symptoms is 10 μg/day; the tolerable maximum uptake is 330 to 450 μg/day (13-15).
Selenium is being actively studied for its role as an antioxidant and its effects on the endocrine system in children. While deficiencies are uncommon in children who maintain a healthy diet, nutritional intake may not always meet their needs, particularly during periods of increased demand, such as adolescence (16). Children on specialized diets are at a higher risk of Se deficiency (17). Research frequently focuses on Se's impact on various aspects of the child's endocrine system. Beyond its association with thyroid diseases, Se is thought to influence obesity, Type 1 diabetes mellitus, polycystic ovary syndrome, and body composition in conditions like syndromic disorders (18-27). Its effects on pancreatic and thyroid cancer have been investigated in adult patients, while its impact on the reproductive system has been studied in animal models (28, 29). It is known that Se supplements are effective in the course of autoimmune thyroiditis. Studies on this subject have also gained momentum in pediatric patients (30).
2. Objectives
However, the stage of the disease, dose, and how long Se supplementation will be effective and sufficient are still being investigated. Our study investigated the effect of short-term and low-dose Se supplementation in pediatric patients with autoimmune thyroiditis who were detected to have early-stage, low-titer autoantibody positivity.
3. Methods
3.1. Patients
The study retrospectively evaluated 54 pediatric cases who applied to the pediatric endocrinology department for various medical conditions and were diagnosed with subclinical hypothyroidism. The data were obtained after examining the database of Ufuk University Pediatric Endocrinology Clinic.
3.2. Exclusion Criteria
Patients included in the study showed normal range complete blood count, folate, vitamin B12, ferritin, and vitamin D results. Patients with dyslipidemia, malignancies, other endocrinological problems, and chronic diseases were excluded.
3.3. Study Design
The patients were divided into two groups: patients with thyroid dysfunction and diagnosed with AITD (group 1, n = 28), and patients with thyroid dysfunction but not diagnosed with AITD (group 2, n = 26). Selenium deficiency was determined according to serum Se levels by age for the Turkish pediatric population (31). Table 1 shows the normal serum Se levels by age for the Turkish pediatric population (31). Selenium supplementation for children was set at 50 mcg, which falls within the tolerable limit range for all age groups (32-34). All cases with Se deficiency were given 50 mcg/day of Se oral replacement therapy for a month before follow-up. Since the study design was retrospective, the sample size was not determined, and power analysis was not performed. Still, the number of cases was in a similar range to studies examining the effect of Se (35).
| Variables | Se Concentration (mcg/L) |
|---|---|
| Age group | |
| 2 - 6 (mon) | 78.5 ± 8.1 (65.6 – 89.8) |
| 7 - 12 (mon) | 87 ± 16.4 (62.4 – 113.9) |
| 1 - 2 (y) | 87.5 ± 11.5 (66.7 – 111.3) |
| 3 - 6 (y) | 90.3 ± 12.6 (72.2 – 116.4) |
| 7 - 13 (y) | 91.2 ± 10.3 (76.4 – 111.1) |
| Total mean SD (range) | 81.1 ± 12.4 (62.4 – 116.4) |
3.4. Data Sources/Measurement
Findings obtained from detailed medical histories, anthropometric evaluations, physical examinations, and thyroid examinations (WHO UNICEF) were analyzed. Thyroid function tests, thyroid autoantibodies (anti-TG, anti-TPO), spot iodine in urine, serum Se levels, and thyroid ultrasound (USG) were evaluated. Thyroid function tests and thyroid autoantibody levels were analyzed in IU/mL via chemiluminescence immunoassay (CLIA), whereas serum Se levels were analyzed in mcg/L via atomic absorption spectrophotometer. Thyroid USG was performed with the use of high-resolution 7.5 MHz probes.
Subclinical hypothyroidism diagnoses were made for cases with elevated thyroid stimulant hormone (TSH) levels and normal sT4 levels (prepubertal TSH: 0.6 - 5.5 mIU/L, sT4: 0.8 - 2.2 ng/mL; pubertal TSH: 0.5 - 4.8 mIU/L, sT4: 0.8 - 2.3 ng/mL) (12). Autoimmune thyroid disease (AITD) diagnoses were made with ultrasonographic findings of heterogeneous echotexture accompanied by elevated titers of thyroid antibodies: Anti-TPO (N: 0 - 5.61 IU/mL) and Anti-TG (N: 0 - 4.11 IU/mL).
3.5. Statistical Analyzes
All statistical analyses were performed using the IBM statistical package for the social sciences (SPSS) software for Windows, version 16.0. All data are expressed as mean ± standard deviation with a 95% confidence interval (CI). For each continuous variable, normality was evaluated using the Shapiro-Wilk test. The Mann-Whitney U test was performed for non-normally distributed data, and Student's t-test was performed for normally distributed data. The patients' thyroid function test results before and after Se replacement treatment were analyzed using the paired t-test or Wilcoxon test. A P-value below 0.05 was considered statistically significant.
4. Results
A total of 54 cases, 33 girls (61.1%) and 21 boys (38.9%), aged 2-17 years (total mean: 9.87 ± 4.1 years; group I mean: 10.53 ± 3.45 years; group II mean: 9.52 ± 4.6 years) were included in the study. Twenty-eight cases (group I) were determined to have Se deficiency and elevated thyroid autoantibody levels. The distribution of patients between groups is shown in Figure 1.
In all of the cases, 26 (53.1%) were prepubertal and 23 (46.9%) were pubertal. The patient group (group I) consisted of 18 girls (64.3%) and 10 boys (35.7%); 10 were prepubertal (41.7%) and 14 were pubertal (58.3%). The control group (group II) consisted of 15 girls (57.7%) and 11 boys (42.3%); 16 cases were prepubertal (64%) and 9 (36%) were pubertal. There was no significant difference between sex and puberty parameters according to the chi-square test.
Patients were grouped according to their Body Mass Index (BMI) as normal (5 - 85 percentile), obese (> 95 percentile), overweight (85 - 95 percentile), and malnourished (< 5 percentile). A total of 10 cases (18.5%) were obese, 5 cases (9.25%) were overweight, and 10 cases were malnourished (18.5%). In the patient group, 8 children (28.57%) were obese, 2 children (7.1%) were overweight, and 5 children were malnourished (17.85%). In the control group, 2 children (7.6%) were obese, 3 children (11.5%) were overweight, and 5 children (19.23%) were malnourished.
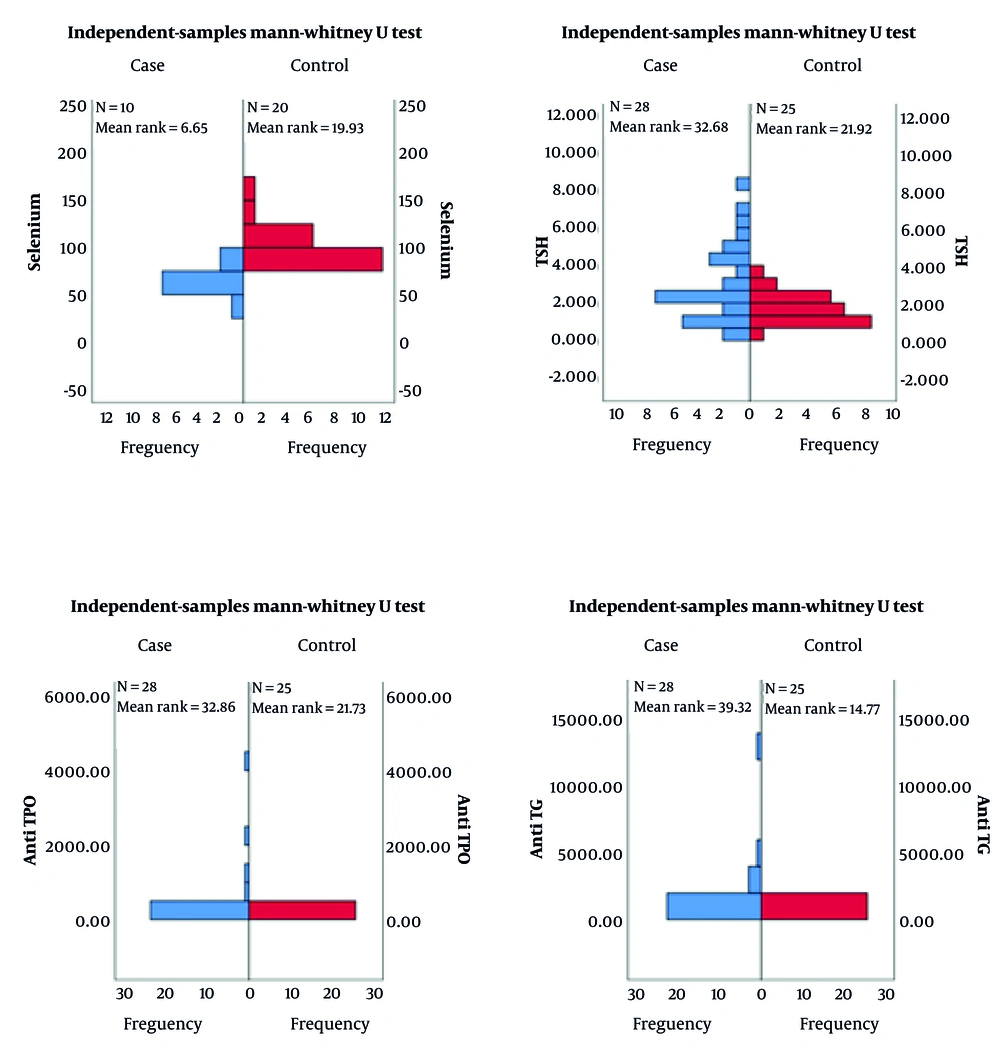
Selenium deficiency was accompanied by elevated anti-TG and anti-TPO levels in group I (51.8%). Selenium levels were normal by age for 26 patients in group II (48.2%) with negative thyroid antibodies. Table 2 shows the mean levels of free T4 (fT4), TSH, anti-TG, anti-TPO, spot iodine in urine, and serum Se levels. The Mann-Whitney U test showed a remarkably significant difference between the two groups for Se levels (P < 0.001), anti-TG (P < 0.009), anti-TPO (P < 0.001), and TSH levels (P < 0.012). Group I had notably low Se levels in contrast to high TSH, anti-TG, and anti-TPO levels. The distribution of Se, TSH levels, and thyroid autoantibodies of the groups are shown in Figure 2.
| Parameters | Case (n = 28) | Control (n = 26) | Total (n = 54) |
|---|---|---|---|
| fT4 | 1.02 ± 0.14 | 1.06 ± 0.18 | 1.04 ± 0.15 |
| TSH | 3.08 ± 2.06 | 1.67 ± 0.78 | 2.40 ± 1.72 |
| Anti-TG | 886.08 ± 2543.87 | 1.06 ± 0.84 | 459.96 ± 1869.7 |
| Anti-TPO | 324.85 ± 909.24 | 0.55 ± 0.62 | 168.70 ± 669.2 |
| Se | 64.40 ± 14.19 | 100.70 ± 20.1 | 88.60 ± 26.3 |
| Spot iodine in urine | 12.517 ± 5.60 | 13,48 ± 6.8 | 13.002 ± 6.2 |
Mean Free T4, Thyroid Stimulant Hormone, Thyroglobulin Autoantibodies, Thyroid Peroxidase Autoantibodies, Serum Selenium and Spot Iodine in Urine levels a
The Spearman's correlation showed a negative correlation (r = -0.762, P = 0.010) between Se deficiency and elevated anti-TPO levels. The control group showed a positive correlation (r = 0.455, P = 0.044) between serum Se and fT4. Statistically significant regression was observed in anti-TG (P = 0.006) and anti-TPO (P < 0.001) levels three months after oral Se replacement therapy (50 mcg/day for a month). Table 3 shows the thyroid function test results of group I before and after Se replacement therapy.
| Parameters | Before Treatment | After Treatment | P-Value |
|---|---|---|---|
| fT4 | 1.02 ± 0.14 | 1.02 ± 0.17 | 0.45 |
| TSH | 3.08 ± 2.06 | 3.06 ± 1.57 | 0.96 |
| Anti-TG | 886.08 ± 2543.87 | 786.23 ± 2302.53 | 0.00 |
| Anti-TPO | 324.85 ± 909.24 | 281.42 ± 765.12 | 0.00 |
Mean Free T4, Thyroid Stimulant Hormone, Thyroglobulin Autoantibodies, Thyroid Peroxidase Autoantibodies Levels of the Group I Before and After Selenium Replacement Therapy a
5. Discussion
Studies have shown that Se has positive effects on children's health. Therefore, epidemiological studies, including Se intake and serum Se concentrations, are needed to determine possible Se deficiency in infants and preschool children (36). The level of Se intake in different diets, the dose, and the duration of replacement that should be used when deficiency is detected are still being investigated. The tolerable Se intake in children is between 45 - 400 mcg, depending on the age range. When geological origin and intake from natural sources are considered, it should not be forgotten that high doses and long-term treatments may exceed the safe range and are cytotoxic and genotoxic (33). Although no adverse effects have been reported in the studies, we have examined the effects of low-dose short-term treatment in children, who are a sensitive population, and observed that the effect is sufficient in the short term. Combined Se preparations are also being investigated, and their effects are being demonstrated in adult autoimmune thyroiditis cases, and the effects of combined preparations on rendering children euthyroid is another subject that can be examined (37, 38).
Selenium may help protect against cancer by reducing the impact of toxic metals through antioxidant processes. The levels of trace elements such as zinc and Se in serum and thyroid tissue are also worth investigating in AITDs and thyroid cancer (39, 40). Zagrodzki et al. found that serum Se levels and plasma GPx activity were much lower than in the control group in children aged 7 - 16 living in an endemic iodine-deficient region in Poland. There was also a significant statistical difference in Se deficiency and fT4 and TSH between male and female genders in cases, indicating that Se and iodine deficiencies are affected by gender (18). Another study conducted by Celik et al. with children from Turkey's endemic iodine-deficient region analyzed whether or not iodine deficiency was accompanied by Se, zinc, copper, or molybdenum deficiency. Selenium deficiency was shown to be in correlation with iodine deficiency. It was emphasized that Se deficiency should be taken into consideration for cases of iodine deficiencies in regions where endemic goiter is prevalent (41).
A study by Keshteli et al. with 2331 Iranian schools did not show a correlation between urine iodine concentration and Se levels. However, goiter was still highly prevalent among these patients. Girls and boys with goiters showed lower Se levels as opposed to their peers who did not. In addition, the need for studies on the deficiency of micronutrients and the role of goitrogens was emphasized (42). Our study was not conducted in an area with endemic iodine deficiency. None of our patients had iodine deficiency. The correlation between iodine and micronutrient deficiency is open to investigation and discussion. Nevertheless, it has been observed that Se deficiency is frequently seen outside areas with endemic iodine deficiency. Therefore, it is very important to check its level in suspected patients. Patients who apply to pediatric endocrinology clinics should be screened for micronutrient parameters. In addition, no statistical difference was found between the two groups in our study according to gender.
Recent studies have tried to elucidate the role Se replacement therapy has in the physiology of Hashimoto's thyroiditis and the formation of thyroid gland enzymes (43). It is assumed that Se replacement therapy may be beneficial for cases with both Hashimoto's thyroiditis and Se deficiency by lowering antibody levels and the required dosage of levothyroxine therapy (44). Our study showed that Se replacement had a positive contribution to the course of the disease in euthyroid patients with low levels of autoantibodies. Kyrgios et al. studied the effects of high-dose organic Se. The study concluded that the Se group showed a much more statistical significance in lowering anti-TG levels in comparison to the placebo group (45). Pediatric Se levels must be evaluated according to the age-based reference range set down by the respective country the study was conducted in. Patients diagnosed with Se deficiency should receive supplement therapy suitable for their age and benefit from Se's anti-inflammatory effects on stopping the progression of autoimmune diseases in their early stages.
5.1. Limitations
In our retrospective study, the number of participants was limited due to the study design. It would be beneficial to conduct a follow-up study with a larger sample size using a double-blind, placebo-controlled methodology. We were unable to investigate the effects of oral Se replacement therapy at different doses and durations. It would be helpful to explore the long-term effects of short-term, low-dose Se treatment over periods of 6 months and 1 year. The study focused on Turkish children and did not implement any specific dietary restrictions. Future research could examine the impact of low-dose, short-term treatment in diverse populations with varying dietary habits.
5.2. Conclusions
The data from our study indicate that Se replacement may have positive effects, especially in the early stages of AITD with low titer antibody elevation. This study highlights the necessity for Se analysis among pediatric AITD cases. Therefore, we believe that Se levels should be screened after oral replacement therapy. Both our study and scientific literature reviews show the impact of Se deficiency on AITD and other autoimmune diseases. We believe it is essential to study and elucidate the effects that Se and other trace elements may have on pediatric AITD cases.