1. Background
Oxygen is essential for cellular and metabolic functions. The transition of the neonate from intrauterine to extrauterine life represents a critical period during which oxygen transport changes significantly and depends on factors such as alveolar ventilation, hemoglobin concentration, and hemoglobin affinity for oxygen (1). The oxygen dissociation curve, a sigmoidal diagram showing the oxygen saturation of hemoglobin at different partial pressures of oxygen (PO2), is an important tool for assessing oxygen transport dynamics. The P50 value, which indicates the oxygen tension at which hemoglobin is 50% saturated, is a clinically relevant parameter that is influenced by factors such as intracellular pH, PCO2, temperature, electrolyte concentration, organic phosphates (such as 2,3-DPG), and the type of hemoglobin. Fetal hemoglobin (HbF) has a higher affinity for oxygen than adult hemoglobin (HbA), resulting in a lower P50 that improves oxygen uptake in the low oxygen tension environment of the placenta. While the fetal P50 value at birth is around 19 mmHg, this value is around 27 mmHg in normal adults. This transition occurs gradually, with neonatal P50 levels reaching adults at 4 - 6 months of age. Fetal blood, which reflects the highest oxygen tension (typically below 30 mmHg), provides a unique insight into fetal oxygenation. At these oxygen levels, fetal blood reaches 6-8% higher oxygen saturation than maternal blood, which promotes effective oxygen transfer to the fetus (2, 3). Therefore, monitoring P50 levels in neonates can provide insight into subtle hypoxic conditions that may go undetected. Meconium-stained amniotic fluid (MSAF), characterized by fetal gastrointestinal contents in the amniotic sac, occurs in 5.1% of preterm births, 16.5% of term pregnancies, and 27.1% of post-term pregnancies. Neonates born through MSAF have a higher risk of respiratory distress and neurologic depression at birth, which may indicate underlying chronic hypoxia or pathologic intrauterine conditions such as infection or asphyxia. However, subtle hypoxic states referred to as "silent hypoxia" often go unrecognized without specific markers such as the P50 (4, 5). The term "silent hypoxemia," originally described in adult critical care literature, refers to a clinical state where patients experience significant tissue-level hypoxia without exhibiting typical respiratory distress symptoms. Clinicians widely recognized this phenomenon during the COVID-19 pandemic when numerous patients had severe hypoxemia undetectable through standard clinical assessments, such as pulse oximetry (6). Although this term is not yet universally established in neonatal medicine, we adopted "silent hypoxemia" in this study to describe subtle, subclinical oxygenation deficits in neonates, which might similarly remain unnoticed using traditional monitoring methods. Thus, our study explores whether the measurement of P50 in umbilical cord blood can serve as an early biomarker for detecting these subtle hypoxic states in neonates born with MSAF.
2. Objectives
This study aimed to investigate the ability of umbilical venous blood gas P50 levels to predict silent hypoxic states in neonates with MSAF.
3. Methods
This prospective observational study was conducted at SBU Istanbul Bağcılar Training and Research Hospital from December 1, 2023, to June 1, 2024. It included newborns delivered by spontaneous vaginal delivery or cesarean section at the gynecology and obstetrics clinic, between 37 and 42 weeks of gestation, as determined by prenatal ultrasound measurements and last menstruation. The gestational age of these infants was confirmed using the new Ballard scoring system, and only births from singleton pregnancies were included. Excluded were multiple pregnancies, preterm births (defined as less than 37 completed weeks of gestation), mothers who had received prenatal steroids for threatened preterm labor, neonates of preeclamptic mothers, mothers who used tobacco and/or alcohol, neonates with congenital anomalies, those who required postnatal resuscitation, and mothers with chronic drug use during pregnancy, chronic disease, or a history of chorioamnionitis. The study group included neonates born with MSAF, while the control group consisted of neonates with no known pathologic birth history (no maternal infections, chorioamnionitis, or resuscitation). Since the study involved newborns and standard clinical monitoring, investigator blinding was not feasible. In the delivery room, data such as maternal age, neonatal sex, mode of delivery, weight, height, head circumference, gestational age, and Apgar scores at 1 and 5 minutes were recorded. Umbilical cord blood gases were analyzed in both groups and the pH, lactate content, base deficit, bicarbonate content, and P50 were documented from the blood gas values. Samples for cord blood gas analysis were collected using the ABL800 FLEX (Radiometer America Inc., Westlake, OH), a fully automated blood gas analyzer that provides quantitative measurements of pH, PCO2, PO2, HCO3, lactate, and P50. With a type I error of 5% and a power of 80%, the required sample size for each group was set at a minimum of 65. Before the study, written informed consent was obtained from the families of the neonates, and ethical approval was granted by the clinical research ethics committee (ethical approval number: 931/23.11.2023).
3.1. Statistical Analysis
The study data were analyzed using SPSS (Statistical Package for Social Sciences) software (version 22 for Windows, SPSS Inc, Chicago, IL, USA). The Shapiro-Wilk test was used to assess the normal distribution of all measured variables. Continuous data that followed a normal distribution were expressed as mean ± standard deviation, while those that did not were expressed as median [1st quartile (Q1)–3rd quartile (Q1)]. Categorical data were expressed in numbers (%). Differences in categorical data between groups were analyzed using the chi-square test or Fisher's exact test. For continuous variables, comparisons were made using the Mann-Whitney U-test or the t-test for independent samples, the latter was considered parametric. Correlation relationships between specific parameters were analyzed using the P50 value with the Pearson or the Spearman test. A multivariate regression analysis was conducted to adjust for potential confounders such as gestational age, birth weight, and maternal conditions. A significance level of P < 0.05 was set for all statistical comparisons.
4. Results
A total of 183 neonates were included in the study. Of these, 68 neonates with MSAF formed the study group and 115 the control group. Demographic characteristics, including maternal age, gestational age, birth weight, and birth length, were comparable between the two groups, with no statistically significant differences observed (P > 0.05). The detailed demographic data are shown in Table 1.
| Variables | Groups | P-Value b | |
|---|---|---|---|
| Study (n = 68) | Control (n = 115) | ||
| Gender | 0.160 c | ||
| Female | 36 (52.9) | 73 (63.5) | |
| Male | 32 (47.1) | 42 (36.5) | |
| Delivery | 0.064 c | ||
| Normal | 50 (73.5) | 69 (60.0) | |
| C/S | 18 (26.5) | 46 (40.0) | |
| Mother age | 29 (24 - 34) | 29 (24 - 33.7) | 0.591 |
| Gestational week | 40 (39 - 40) | 39 (38 - 40) | 0.071 |
| Weight | 3360 (3152 - 3700) | 3245 (3092 - 3500) | 0.067 |
| Height | 51 (49 - 52) | 50 (49 - 52) | 0.188 |
| Head circumference | 35 (34 - 35) | 34.5 (34 - 35) | 0.091 |
| Apgar 1 | 9 (8 - 9) | 9 (8 - 9) | 0.473 |
| Apgar 5 | 10 (9 - 10) | 10 (9 - 10) | 0.368 |
| Perfusion Index | 2.54 (2.1 - 3.1) | 2.5 (2.0 - 3.0) | 0.171 |
Demographic Data a
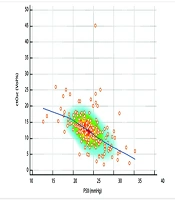
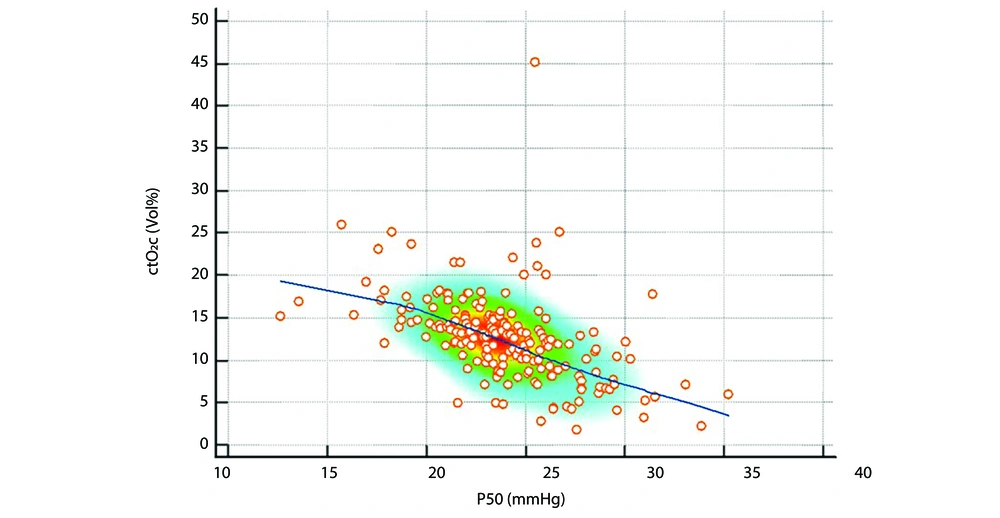
The mean P50 was significantly higher in the MSAF group than in the control group (24.65 ± 3.52 vs. 23.54 ± 3.37, P = 0.036). Lactate levels were also significantly higher in the MSAF group (median: 2.81, Q1 - Q3: 2.22 - 3.50) than in the control group (median: 2.30, Q1 - Q3: 1.94 - 3.20, P = 0.003), indicating an altered oxygen metabolism. Conversely, the median ctO2 was lower in the MSAF group (11.9, Q1 - Q3: 8.4 - 14.0) than in the control group (13.1, Q1 - Q3: 10.4 - 15.3, P = 0.035). However, parameters such as pH and oxygen saturation (SO2) were not significantly different between the groups (P > 0.05). The comparative analyses of the blood gas parameters are shown in Table 2. Correlation analyses revealed significant correlations between the P50 and the blood gas parameters in both groups. In the MSAF group, the P50 was positively correlated with lactate levels (r = 0.39, P = 0.001), indicating a relationship between increased oxygen dissociation and increased lactate levels (Figure 1). Negative correlations were found between the P50 and ctO2 (r = -0.54, P < 0.001), pH (r = -0.53, P < 0.001), and SO2 (r = -0.53, P < 0.001) (Figure 2).
| Variables | Groups a | P-Value b | |
|---|---|---|---|
| Study (n = 68) | Control (n = 115) | ||
| cBase | -2.3 (-4.85 to -1.0) | -2.20 (-3.6 to -0.5) | 0.087 |
| Lactate | 2.81 (2.22 - 3.50) | 2.30 (1.94 - 3.20) | 0.003 |
| P50; mean ± SD | 24.65 ± 3.52 | 23.54 ± 3.37 | 0.036 c |
| ctO2 | 11.9 (8.4 - 14.0) | 13.1 (10.4 - 15.3) | 0.035 |
| pH | 7.32 (7.28 - 7.37) | 7.34 (7.31 - 7.37) | 0.164 |
| PCO2 | 43.7 (38.52 - 50.97) | 42.6 (38.7 - 47.0) | 0.204 |
| SO2 | 53.9 (37.27 - 71.32) | 61.3 (48.9 - 74.3) | 0.084 |
| HCO3(P)c; mean ± SD | 22.04 ± 2.40 | 22.69 ± 2.47 | 0.086 c |
P50 and Blood Gas Parameters
Correlation between P50 and lactate levels: This scatter plot illustrates the correlation between P50 and lactate levels in neonates. The positive correlation (r = 0.39, P = 0.001) observed in the meconium-stained amniotic fluid (MSAF) group suggests that increased oxygen dissociation is associated with elevated lactate levels, indicating impaired oxygen utilization. The color density represents data clustering.
Correlation Between P50 and oxygen content (ctO2): This scatter plot depicts the relationship between P50 and ctO2 in neonates. A significant negative correlation (r = -0.54, P < 0.001) was observed, suggesting that higher P50 values are associated with reduced oxygen content, reflecting decreased systemic oxygen availability. The density distribution highlights areas with higher data concentration.
These results suggest that higher P50 levels in the MSAF group were associated with hypoxemia and decreased oxygen delivery. Similar but less pronounced correlations were observed in the control group. P50 was positively correlated with lactate levels (r = 0.23, P = 0.011) and negatively correlated with ctO2 (r = -0.62, P < 0.001), pH (r = -0.48, P < 0.001), and SO₂ (r = -0.53, P < 0.001). These results support the role of P50 as a marker for oxygen dissociation. A summary of the correlation results can be found in Table 3. Multivariate regression analysis demonstrated that P50 remained significantly associated with lactate and oxygen content (P < 0.01) even after adjusting for gestational age, birth weight, and maternal conditions. These findings indicate that P50 is independently linked to neonatal oxygenation status, reinforcing its potential role as a biomarker for silent hypoxia. To assess the potential influence of maternal factors, we included smoking, early membrane rupture (EMR), and urinary tract infection (UTI) as covariates in the regression model. However, the analysis revealed that none of these maternal variables had a statistically significant effect on P50 levels (smoking: β = 5.19, P = 0.103; EMR: β = 1.30, P = 0.423; UTI: β = -0.15, P = 0.896). These results suggest that P50 variations are primarily driven by neonatal oxygen transport mechanisms rather than maternal conditions. Consequently, neonatal parameters should be prioritized when evaluating oxygen dissociation dynamics in neonates with MSAF (Table 4).
| Variables | Study Group | Control Group | ||
|---|---|---|---|---|
| r-Value (Correlation Coefficient) | P-Value a | r-Value (Correlation Coefficient) | P-Value a | |
| cBase | -0.001 | 0.991 | -0.98 | 0.298 |
| Lactate | 0.39 | 0.001 | 0.23 | 0.011 |
| ctO2 | -0.54 | < 0.001 | -0.62 | < 0.001 |
| pH | -0.53 | < 0.001 | -0.48 | < 0.001 |
| PCO2 | 0.50 | < 0.001 | 0.47 | < 0.001 |
| SO2 | -0.53 | < 0.001 | -0.53 | < 0.001 |
| HCO3(P)c | 0.010 | 0.936 b | -0.002 | 0.987 b |
Correlation Between P50 and Blood Gas Parameters
| Variables | β (Coefficient) | Std. Error | t-Value | P-Value |
|---|---|---|---|---|
| Constant | 23.60 | 0.41 | 58.12 | < 0.001 |
| Smoking | 5.19 | 3.14 | 1.65 | 0.103 |
| EMR | 1.30 | 1.61 | 0.81 | 0.423 |
| UTI | -0.15 | 1.18 | -0.13 | 0.896 |
Maternal Factors Regression Results
5. Discussion
The relationship between P50 level and hypoxemia in neonates with MSAF reflects the complicated physiologic responses to perinatal hypoxia (7). P50 levels were significantly higher in the MSAF group than in the control group, indicating a compensatory adaptation of hemoglobin to facilitate oxygen release under conditions of reduced oxygen availability. Although the differences in P50 and lactate levels between groups were statistically significant, their clinical implications remain uncertain. P50 may serve as an early indicator for oxygenation disturbances in neonates with MSAF, allowing for timely intervention before overt hypoxemia develops. However, further studies are required to determine whether P50-guided monitoring improves neonatal outcomes.
P50 is a critical parameter reflecting the affinity of hemoglobin for oxygen and its ability to release oxygen to tissues. In neonates, P50 levels are influenced by the predominance of HbF, which has a higher affinity for oxygen compared to HbA. This allows for efficient oxygen transfer from maternal to fetal circulation. In our study, P50 levels were significantly higher in neonates with MSAF compared to controls, indicating potential alterations in oxygen delivery dynamics. While an increase in P50 is generally associated with neonatal hypoxia and adaptive oxygen transport mechanisms, there is no universally established threshold to define hypoxia based solely on P50 values. Variability in P50 levels suggests that it should be interpreted cautiously and in conjunction with other clinical parameters. Therefore, monitoring P50 in neonates with MSAF may provide additional insights into oxygenation status, but further studies are needed to clarify its clinical implications and determine whether it should be incorporated into routine blood gas assessments.
The higher lactate levels observed in the MSAF group also suggest the presence of hypoxemia (8). Lactate accumulation due to anaerobic metabolism is a marker for inadequate tissue oxygenation. The positive correlation between the P50 value and the lactate level (r = 0.39, P = 0.001) illustrates the metabolic consequences of insufficient oxygen supply. Conversely, the negative correlations between P50 and ctO2 (r = -0.54, P < 0.001), pH (r = -0.53, P < 0.001), and SO2 (r = -0.53, P < 0.001) indicate that higher P50 levels are associated with decreased oxygenation and worsening acidosis, particularly in neonates with MSAF. Multivariate regression analysis confirmed that P50 remained significantly associated with lactate and oxygen content (P < 0.01) even after adjusting for gestational age, birth weight, and maternal conditions. These results suggest an independent relationship between P50 and oxygenation status in neonates with MSAF, supporting its potential role as a biomarker for subclinical hypoxia.
These findings are clinically significant because standard oxygenation indices such as pulse oximetry or oxygen saturation may not fully capture the extent of hypoxemia in MSAF. For example, while pH and SO2 levels were not significantly different between groups, the increased P50 and lactate levels in the MSAF group indicated silent hypoxia. This discrepancy emphasizes the need for advanced monitoring methods to comprehensively assess the dynamics of oxygen transport. Mandruzzato et al. (9) highlighted the importance of detailed monitoring in IUGR fetuses, which, like those born with MSAF, are at risk of unrecognized hypoxemia, further emphasizing the need for additional monitoring methods.
Previous studies have highlighted the role of endothelin-1 in hypoxic conditions and have linked MSAF to an increased risk of hypoxemia (10). Prolonged fetal exposure to MSAF has been associated with activation of inflammatory pathways, including elevation in pro-inflammatory cytokines such as interleukin-6 and TNF-alpha, which may contribute to increased oxidative stress (5). Such inflammatory responses could disrupt oxygen transport mechanisms, potentially altering erythropoiesis and hemoglobin affinity for oxygen. Further research is necessary to better understand these underlying pathophysiological mechanisms and their implications for clinical management. However, to our knowledge, this is the first study to examine the relationship between the P50, blood gas parameters, and hypoxemia in neonates with MSAF. Our study fills an important gap in the literature by examining the relationship between P50 levels, blood gas parameters, and hypoxemia in neonates with MSAF and offers new insights into the mechanisms of adaptation to hypoxemia in this vulnerable population.
Rao et al. (11) also discussed how MSAF, particularly when associated with histologic chorioamnionitis, may exacerbate neonatal morbidity due to impaired oxygenation and increased inflammation, supporting our findings regarding altered oxygen transport dynamics. Despite the compensatory increase in oxygen dissociation, the lower ctO2 observed in the MSAF group underscores the systemic effects of hypoxemia. Even when hemoglobin adapts to release oxygen more readily, the reduced total oxygen availability limits its ability to meet metabolic demands, as evidenced by increased lactate levels. Recent evidence suggests that neonates experiencing perinatal stress, including those with meconium exposure, may develop persistent metabolic acidosis despite compensatory mechanisms due to altered oxygen utilization dynamics (4). This is consistent with a compensatory mechanism, which is helpful but cannot fully compensate for the metabolic consequences of reduced oxygen-carrying capacity.
Elsokkary et al. (12) found that increased nucleated red blood cells (RBCs) in cord blood of MSAF neonates reflect the body's efforts to improve oxygen delivery under stress, which is consistent with our observation of compensatory mechanisms, such as increased P50. Elevated lactate and P50 levels, and decreased ctO2, may indicate significant stress and the need for urgent intervention in neonates (13). Studies have also shown that neonates born with MSAF have an increased risk of adverse outcomes, including respiratory distress syndrome and hypoxic-ischemic encephalopathy, which are often reflected in abnormal blood gas levels (14). Satomi et al. (15) also pointed out the increased risk of respiratory distress syndrome in neonates with MSAF and emphasized the importance of early recognition and treatment of hypoxemia in these cases.
Our results have implications for clinical practice. The strong correlations between the P50 and markers of acidosis, such as lactate and pH, suggest that the P50 could serve as a valuable additional marker for assessing oxygenation status in neonates, particularly those with MSAF. Incorporating P50 assessments into routine monitoring could improve the detection of silent hypoxemia, allow timely intervention, and reduce the risk of complications associated with prolonged hypoxemia. Bachman et al. (16) have discussed how thresholds for oxygenation alarms in neonatal care could be adjusted based on factors such as the P50 score, which would provide a more accurate way to monitor oxygen status and intervene early.
This study has some limitations. The lack of long-term follow-up data prevents us from assessing whether P50 monitoring translates into better neonatal outcomes. Additionally, our findings are based on a single-center study with a relatively small sample size, which may limit generalizability. Future multicenter studies with longitudinal follow-up are required to validate our findings and explore the clinical utility of P50 as a routine neonatal monitoring parameter. Future studies should investigate whether routine P50 assessment in neonates with MSAF could lead to earlier recognition of oxygenation disturbances and improved clinical outcomes. Additionally, exploring the role of inflammatory mediators and oxidative stress in P50 alterations could provide deeper insights into the pathophysiology of silent hypoxia in neonates. Recent neonatal research suggests that advanced oxygenation monitoring strategies, including P50 evaluation, may improve early detection and intervention for high-risk neonates (5).
5.1. Conclusions
In conclusion, the significantly higher P50 values observed in neonates with MSAF may be due to adaptive changes in oxygen transport dynamics in response to hypoxemia, with increased lactate levels and decreased oxygen content. Differences in this adaptation among neonates or the fact that some neonates have not fully developed these adaptation mechanisms may cause mild hypoxia to become clinically severe. Therefore, further research is needed to confirm the results and explore the potential of P50 as a clinical tool to monitor and treat hypoxemia in neonates.