1. Background
Congenital heart disease (CHD), the most common birth anomaly in children, refers to structural abnormalities of the heart or major intrathoracic blood vessels that develop during fetal growth. It is the leading cause of mortality in infants with congenital malformations (1). Acyanotic congenital heart diseases (aCHDs), such as ventricular septal defect (VSD), atrial septal defect (ASD), patent ductus arteriosus (PDA), coarctation of the aorta (COA), and valvular and supravalvular aortic stenosis (AS), constitute the majority of congenital cardiac anomalies in children (2-4). Previously, the incidence of CHD was estimated at 5 - 8 per 1000 live births. However, advancements in echocardiography have led to the detection of milder cases, increasing the reported incidence to 8 to 12 per 1000 live births. Nevertheless, these figures still vary depending on the timing and intensity of diagnostic efforts (5). A recent meta-analysis reported an overall CHD prevalence of 2.5 per 1000 live births and children in Iran (6).
Advancements in cardiac surgical techniques and neonatal intensive care have significantly improved long-term survival rates for CHD patients (7), enabling many to survive into adulthood (8). As early surgical outcomes have become more successful, the focus has shifted from addressing cardiac issues to managing acquired cardiovascular and systemic complications (9). Consequently, there is now greater emphasis on the longer-term quality of life for these patients, particularly regarding cognitive development and neurodevelopmental disorders (2, 10-13), which are the most prevalent and disabling long-term complications (11, 14, 15).
Neurodevelopmental impairment in CHD patients is multifactorial (16-19), with surgical interventions also influencing neurocognitive outcomes. Considering the increased vulnerability of the brain of children with CHD, they may experience worse neurocognitive function compared to their healthy peers (20). Neurodevelopmental disorders are characterized by deficits in cognitive, social, and academic functioning that become evident early in development (21). Children with moderate and severe CHD who have undergone cardiopulmonary bypass (CPB) are particularly at risk for impairments in cognitive performance, motor skills, social interaction, language, attention, impulsivity, and executive function (22-24). These deficits can negatively impact psychosocial, behavioral, and academic performance, quality of life, and independence in adulthood (11, 24).
Various studies have been conducted on the neurodevelopment of children with CHD and the factors influencing it (2, 11, 15, 20). One such study was carried out by Ozmen et al. in 2016 on 132 children with aCHD to evaluate neurodevelopment in these patients during the preschool period and identify the factors affecting it. They concluded that neurodevelopmental disorders in children with aCHD are more prevalent than in the general population and are influenced by various factors, including socioeconomic issues, health complications during the perinatal period, and iron deficiency (3).
2. Objectives
This study aims to evaluate the neurodevelopmental outcomes of children with congenital acyanotic cardiopathy who have undergone congenital heart surgery.
3. Methods
3.1. Study Population
We performed a retrospective cohort study at Rajaie Cardiovascular Medical and Research Center, Tehran, Iran, from 2019 to 2022. Fifty children aged 4 to 69 months with acyanotic and non-complex congenital heart disease, including VSD, ASD, COA, pulmonary stenosis (PS), AS, and PDA, who required corrective open-heart surgery, and 50 healthy children as a control group were recruited for this study. The control group consisted of children in the same age range as the case group who were referred to the Pediatric Cardiology Department and Clinic, Shahid Rajaei Hospital, Tehran, Iran, for further evaluation due to reasons such as the detection of a heart murmur during auscultation. Upon examination at this center, the murmur was diagnosed as innocent, and the child was confirmed to be free of any cardiac issues following evaluation and examination by a specialist.
3.2. Inclusion and Exclusion Criteria
Children visiting Rajaie Cardiovascular Medical and Research Center, aged over 2 months and under 69 months, with a birth weight over 2000 grams and normal physical growth, were included in this study. Children with cyanotic congenital heart disease or complex cardiac conditions, those with clinically recognizable genetic syndromes, metabolic disorders, immune deficiencies, CNS developmental anomalies, any acquired perinatal insults such as hypoxic-ischemic encephalopathy, stroke, prematurity, and sepsis, or with a history of seizures or use of anticonvulsant drugs, those with a family history of genetic, metabolic, or neurological disorders, and children with chronic non-cardiac diseases (renal, endocrine, etc.) were excluded from the study. Additionally, if unexpected complications occurred during the operation, such as hypoxia, the need for reoperation, prolonged surgery time (pump time) beyond the estimated duration for each patient, or an unusual course in the intensive care unit after surgery, they were excluded from the study.
3.3. Main Outcome Measured
Initial information about the child, including age, gender, history of other diseases, surgeries or interventions, parents' education level, parental consanguinity, economic status, and feeding type, was collected. The diagnosis of CHD was made by an academic pediatric cardiologist. Furthermore, the Modified Denver Developmental Screening Test II (DDST II) was used for the assessment of developmental milestones in these children before and up to six months post-surgery. The validity and reliability of this test have been confirmed and are considered standard for the Iranian population in previous studies (25). Gross motor, fine motor, speech (language), and psycho-social aspects were assessed in all control and case subjects before surgery, but post-operative tests were conducted only on those whose surgical course appeared to be without complications. We categorized the test results as normal or delayed if a red flag was passed in one aspect or if the yellow zone was reached in two aspects, based on the definition and subsequently confirmed through neurological examination by an expert pediatric neurologist. The test results were then compared to the attained neurodevelopmental growth in children with aCHD to age-matched controls without CHD.
3.4. Assessment Tool
The DDST II identifies cognitive and behavioral issues in children aged 2 to 72 months. It evaluates four areas: Personal-social, fine motor, language, and gross motor skills. The test requires no special training, takes about 20 minutes, and can be administered by someone with brief training and experience with children. Observations are recorded on a chart adjusted for age, including adjustments for prematurity. Difficulties in tasks may indicate developmental delays, warranting further evaluation (26).
3.5. Data Analysis Method
Descriptive statistics, including means, standard deviations, and percentages, were calculated for all variables. Comparative analyses between the case and control groups were performed using chi-square tests for categorical variables and Mann-Whitney U tests for continuous variables. Pre- and post-operation comparisons within the case group were assessed using the Wilcoxon Signed Ranks Test. The normality of the data was tested using the Kolmogorov-Smirnov test. All statistical analyses were conducted using SPSS software (version 16, IBM Inc., Chicago, IL, USA, 2007), and a P-value of < 0.05 was considered statistically significant.
4. Results
In this study, we included data involving 50 patients diagnosed with aCHD who required corrective open-heart surgery with no known neurological deficits and 50 healthy children as the control group. The mean age of the 50 participants in the case group at the time of surgery was 30.28 ± 22.24 months, while the mean age of the 50 participants in the control group during the examination at the same time was 28.44 ± 19.69 months. There was no significant difference in age between the two groups (P = 0.967). Additionally, the birth weight of 41 participants in the case group and 16 participants in the control group was checked to assess eligibility criteria and confirm the absence of preterm birth. The mean birth weight was 3.09 ± 0.38 kg and 2.94 ± 0.86 kg, respectively, with no significant difference between the two groups (P = 0.803).
Subsequently, other information regarding the child, including demographics, parenting, and socioeconomic variables of participants, was examined in both groups and is presented in Table 1. As shown in Table 1, variables including gender, type of infant feeding, the family’s economic status from their own perspective, parental education level, and parental consanguinity were examined. Among these, only the difference in parental education level between the two groups was significant. A significant difference was found between the groups (P = 0.047), indicating a potential association between maternal education level and group classification. Similarly, a significant difference in paternal education level between the groups was observed (P = 0.015).
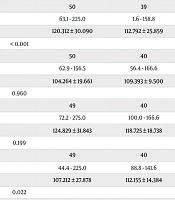
The results of the modified DDST II, which was used for the assessment of developmental milestones in case and control groups pre-operation and also case pre- and post-operation, are shown in Table 2. Pre-operatively, children in the aCHD group scored significantly lower in fine motor adaptive skills (P < 0.001) and total developmental scores (P = 0.022) compared to the control group. However, no statistically significant postoperative improvements were observed in the developmental domains assessed by DDST II in the case group, and the total developmental scores of the case group remained consistent pre- and post-operation (P = 0.994), indicating that corrective surgery did not lead to immediate neurodevelopmental improvement.
| Variables | Case | Control | P-Value b |
|---|---|---|---|
| Gender | 0.545 | ||
| Female | 30 (60) | 27 (54) | |
| Male | 20 (40) | 23 (46) | |
| Total | 50 (100) | 50 (100) | |
| Feeding type | 0.222 | ||
| Breast feeding | 30 (61.2) | 20 (71.4) | |
| Feeding with formula | 11 (22.4) | 2 (7.2) | |
| Mixed feeding | 8 (16.3) | 6 (21.4) | |
| Total | 49 (100) | 28 (100) | |
| Economic status | 0.156 | ||
| Bad | 2 (20) | 2 (8.3) | |
| Average | 4 (40) | 18 (75) | |
| Good | 2 (20) | 4 (16.7) | |
| Total | 10 (100) | 24 (100) | |
| Mother’s education | 0.047 | ||
| Elementary | 12 (24) | 0 (0) | |
| Middle school | 5 (10) | 5 (14.7) | |
| Diploma | 23 (46) | 17 (50) | |
| Associate | 4 (8) | 3 (8.8) | |
| Bachelor | 4 (8) | 7 (20.6) | |
| Master | 2 (4) | 2 (5.9) | |
| Total | 50 (100) | 34 (100) | |
| Father’s education | 0.015 | ||
| Elementary | 11 (22) | 0 (0) | |
| Middle school | 13 (26) | 7 (20.6) | |
| Diploma | 17 (34) | 15 (44.1) | |
| Associate | 0 (0) | 2 (5.9) | |
| Bachelor | 8 (16) | 6 (17.6) | |
| Master | 0 (0) | 3 (8.8) | |
| Doctorate | 1 (2) | 1 (2.9) | |
| Total | 50 (100) | 34 (100) | |
| Family-consanguinity | 0.169 | ||
| Yes | 1 (2) | 4 (8) | |
| No | 49 (98) | 46 (92) | |
| Total | 50 (100) | 50 (100) |
a Values are expressed as No. (%).
b Based on the chi-square test.
| Variables | Pre-Operation | Pre- and Post- Operation | ||||
|---|---|---|---|---|---|---|
| Denver Developmental Screening Test II Items | Case | Control | P-Value a | Case Before Operation | Case After Operation | P-Value b |
| Personal social skills | 0.580 | 0.210 | ||||
| Valid (No.) | 50 | 50 | 50 | 39 | ||
| Min-max | 63.1 - 225.0 | 75.0 - 170.0 | 63.1 - 225.0 | 1.6 - 158.8 | ||
| Mean ± SD | 120.312 ± 30.090 | 121.496 ± 23.014 | 120.312 ± 30.090 | 112.792 ± 25.859 | ||
| Fine motor adaptive skills | < 0.001 | 0.332 | ||||
| Valid (No.) | 50 | 49 | 50 | 40 | ||
| Min-max | 62.9 - 156.5 | 84.6 - 181.8 | 62.9 - 156.5 | 56.4 - 166.6 | ||
| Mean ± SD | 104.264 ± 19.661 | 119.600 ± 17.967 | 104.264 ± 19.661 | 109.393 ± 9.500 | ||
| Language | 0.960 | 0.489 | ||||
| Valid (No.) | 49 | 49 | 49 | 40 | ||
| Min-max | 72.2 - 275.0 | 88.8 - 175.0 | 72.2 - 275.0 | 100.0 - 166.6 | ||
| Mean ± SD | 124.829 ± 31.843 | 121.671 ± 18.766 | 124.829 ± 31.843 | 118.725 ± 18.738 | ||
| Gross motor function | 0.199 | 0.222 | ||||
| Valid (No.) | 49 | 49 | 49 | 40 | ||
| Min-max | 44.4 - 225.0 | 66.6 - 166.6 | 44.4 - 225.0 | 88.8 - 141.6 | ||
| Mean ± SD | 107.212 ± 27.878 | 112.898 ± 22.654 | 107.212 ± 27.878 | 112.155 ± 14.384 | ||
| Total items | 0.022 | 0.994 | ||||
| Valid (No.) | 50 | 50 | 50 | 41 | ||
| Min-max | 91.1 - 206.3 | 90.6 - 154.5 | 91.1 - 206.3 | 85.4 - 147.9 | ||
| Mean ± SD | 114.0 ± 18.594 | 119.277 ± 100 | 114.0 ± 18.594 | 113.606 ± 13.3679 | ||
a Based on the Mann-Whitney U test.
b Based on the Wilcoxon Signed Ranks Test.
5. Discussion
This study aimed to assess the neurodevelopmental outcomes of children undergoing surgery for aCHD in an Iranian population, with a specific focus on the impact of the surgery on developmental skills measured by DDST II. Our findings revealed that patients with aCHD, compared to healthy children, exhibit neurodevelopmental delays, particularly in the domain of fine motor skills. This is consistent with the results of other studies, which indicate developmental delays in children with CHD compared to the healthy pediatric population (2, 11).
Despite expectations of improved developmental outcomes following surgery, our results showed that surgery had no statistically significant effect on the assessed developmental domains, including personal-social, fine motor-adaptive, language, and gross motor skills in patients with aCHD. These findings align with Ozmen et al.’s earlier research using DDST II, which also reported no significant factors influencing gross motor, fine motor, language, or personal-social development in children with aCHD (3). The lack of observed improvement may be attributed to several factors, including the short duration between surgery and post-operative assessment. Neurodevelopmental progress may require a longer follow-up period to capture subtle or delayed improvements in skills. Moreover, the baseline developmental status of the children and potential environmental factors, such as access to rehabilitation or early intervention programs, could influence outcomes.
Prior research by Sarrechia et al. has shown that while surgical interventions address structural cardiac issues, they may not fully resolve developmental challenges. Newborns requiring open-heart surgery are at risk for global developmental delays that may manifest over time. They emphasized the multifactorial nature of these outcomes and recommended monitoring school performance during follow-ups to identify and refer at-risk children (2). Similarly, studies by Sood et al., Wernovsky and Licht, Donofrio and Massaro, and Limperopoulos et al. emphasize that children with CHD remain vulnerable to developmental delays even after surgical correction due to factors like baseline neurodevelopmental status, pre-operative hypoxia, perioperative stress, surgical techniques, and underlying genetic conditions (15, 24, 27, 28). These findings support our conclusion that surgery alone is insufficient for optimal neurodevelopmental progress.
A key finding of this study was the significant association between parental education levels and neurodevelopmental outcomes. In contrast, Ozmen et al. identified no relationship between the mother’s level of education and the mental development of children with aCHD, instead mentioning only the father's low level of education, alongside iron deficiency anemia and receiving incubator care, as contributing factors to the mental development of these children (3). However, our findings are consistent with research by Sood et al. and Feng et al., which highlighted the positive correlation between maternal education levels and developmental performance in children with CHD (24, 29). Bucholz et al. reported that lower maternal education levels were associated with greater delays in communication and problem-solving skills in children with hypoplastic left heart syndrome by age 3, with further delays in problem-solving and fine motor skills observed by age 5 (30).
Children with CHD require ongoing cardiology follow-up, including regular echocardiographic evaluations to monitor for residual defects and determine the need for re-intervention (31-33). However, as highlighted by Mackie et al., lapses in care and missed visits are common in patients with CHD, particularly those from lower socioeconomic backgrounds. These gaps are often linked to limited family education and poor understanding of the necessity for ongoing cardiac surveillance (34). Rao noted that moderate to large VSDs require daily pharmacologic management with agents such as diuretics and after-load reducers to control volume overload (35). Asani et al. found a significant correlation between maternal education and the ability to accurately identify prescribed medications, demonstrating the critical role of health literacy in adherence to treatment regimens (36). Similarly, Xiang et al. reported that parents from lower socioeconomic and educational status tend to have poorer health literacy, which may contribute to increased rates of unplanned hospital admissions and greater reliance on local healthcare resources (37). These underscore the critical role of parental involvement and education in shaping child outcomes, even in the context of medical conditions like CHD. Nevertheless, more in-depth studies are needed to evaluate the effects of parental education level on the long-term outcomes of patients with aCHD.
Other factors, including economic status, consanguinity, feeding type, and past medical history, were not significantly associated with developmental outcomes in this study. While economic status often influences access to healthcare and resources, the lack of a significant association in this study may reflect the relatively homogeneous socioeconomic background of the participants. Likewise, consanguinity, despite its known genetic risks, was not a significant factor in this context. Although Feng et al. identified the lack of exclusive breastfeeding or mixed feeding during the first year after birth, compared to exclusive breastfeeding, as a risk factor influencing poor neurodevelopment (29), no significant difference was found in this study. It may be due to cultural, nutritional, or healthcare-related differences between the Iranian and Chinese populations, as well as potential variations in study design or sample characteristics. Further research is warranted to explore the impact of breastfeeding practices on neurodevelopment across diverse populations.
The findings of this study highlight the importance of educating and empowering parents, particularly in low-resource settings, as a crucial factor in enhancing developmental outcomes in children with aCHD. Similar to findings from Vagha et al.’s study in India on 82 children with CHD, this research underscores the significance of comprehensive care for these children to improve their quality of life (38). Since surgical correction alone may not be sufficient to optimize neurodevelopmental outcomes, additional interventions, such as early developmental screenings and targeted therapies, should be considered.
5.1. Limitations
This study has several limitations. The sample size was relatively small, which may have limited the statistical power to detect subtle differences. Additionally, the short follow-up period may not have been sufficient to observe delayed neurodevelopmental changes. The use of a single tool, DDST II, may have restricted the scope of the developmental assessment, and future studies could incorporate other standardized measures. Furthermore, this study did not assess the role of other therapies, the impact of parental involvement in early rehabilitative therapy, or long-term follow-up, limiting its comparability to studies addressing these aspects.
We suggest future research should include longitudinal studies with larger and more diverse populations to evaluate long-term neurodevelopmental progression in children with aCHD. Investigating the combined effects of surgery, rehabilitation, and family support systems on developmental outcomes could provide valuable insights. Additionally, exploring the role of cultural and environmental factors in shaping neurodevelopmental outcomes in different populations may yield important findings.
5.2. Conclusions
In conclusion, this study highlights the complex interplay of surgical, environmental, and familial factors in determining neurodevelopmental outcomes in children with aCHD. While surgery did not lead to immediate improvements in developmental skills, parental education emerged as a significant contributor to better outcomes. These findings reinforce the need for multidimensional and interdisciplinary care approaches that address both medical and socio-environmental determinants of child development.