1. Background
Damage to the developing brain results in impaired postural control and motor function in cerebral palsy (CP) (1). The location of involvement in CP can be either unilateral or bilateral. Unilateral cerebral palsy (UCP), which affects one side of the body, including the extremities and trunk, represents approximately 30% of all CP cases (2). Bilateral cerebral palsy (BCP) is characterized by the involvement of both sides of the body, including the extremities and torso (3). As the severity of motor impairment increases in CP, postural control is negatively impacted. Although children with UCP tend to have better functional outcomes, their postural control is still affected (4, 5), and they are at risk for developing asymmetric spinal posture (6). While children with CP are born with a normal musculoskeletal system, they are at significant risk for the early development and rapid deterioration of asymmetric postural issues and abnormal spinal movement patterns (7).
In children with CP, abnormal posture may emerge as a result of deficiencies in the spinal alignment process, leading to secondary spinal deformities and movement disorders. Early determination of such abnormalities may help prevent secondary disorders in CP (8). Posture-oriented assessment and intervention programs can play a role in preventing contractures, deformities, pain, and asymmetry (9). Trunk control plays a key role in effective postural control. The stabilization of the trunk enables the effective movement of the head and distal segments, supports the visual and vestibular control of posture, facilitates the development of goal-directed activities and gross motor skills, and allows for the proper alignment of the body for communication and social skills (10, 11). When trunk control is impaired during early life, it can significantly affect overall motor development and, through delayed and limited motor functions, even impact cognitive and emotional development in children. The interaction between the trunk, upper extremities, and head changes with age, depending on maturation. Children are more susceptible to developing spinal deformities than adults due to muscle weakness, which also affects trunk movement and stability (12). Trunk movements are important for maintaining mobility and postural reactions during limb movements (8).
In children with CP, abnormal posture may emerge as a result of deficiencies in the spinal alignment process, leading to secondary spinal deformities and movement disorders (9). The disorder consisting of pathologic muscle tone, increased angle of curvature, and secondary inhibitory vertebral growth disorders in the concavity of the curve predisposes to progressive vertebral and spinal deformity as well as curve stiffness (13). Scoliosis has been reported in 40% of children with UCP (14). Postural deformity may also begin when an individual spends too much time in a particular asymmetrical position (7). Asymmetric muscle activity does not clearly explain deformity patterns; no relationship has been established between the direction of increased muscle tone and the direction of scoliosis (15). Galvis et al. found that the lateral flexion angles of healthy adolescents with scoliosis were similar on both sides. Increased mobility was observed especially in the segments below and above the apex of the scoliotic curve, indicating that the scoliotic spine is flexible and can compensate near the apex (16).
Healthcare professionals need a reliable and valid method to estimate spinal range of motion limitations to support clinical decisions regarding individual prognosis and management (17). Evaluation of lateral mobility on the affected and unaffected side in children with UCP with asymmetric postural involvement will clinically shed light on interventions in terms of providing information about the flexibility of the spine and development of right posture. Posture-oriented assessment and intervention programs can play a role in preventing contractures, deformities, pain, and asymmetry (18).
2. Objectives
There are few studies that describe asymmetric spinal alignment in UCP (6, 14). However, no study has compared spinal alignment and spinal mobility between the affected and unaffected sides in UCP. The present study is to compare spinal posture and lateral spinal mobility between the affected and unaffected sides in children with UCP.
3. Methods
3.1. Participants
This cross-sectional study included 60 children with UCP attending Duzce Gokkusagı and Eregli Gokkusagı special education and rehabilitation centers. Children who met the inclusion criteria and whose parents and themselves voluntarily agreed to participate were included. Based on power analysis, the minimum sample size was determined to be 36, with a 90% power and a 0.05 alpha error margin. Two children were excluded due to scoliosis surgery. Consequently, evaluations were conducted on 58 children with CP, and informed consent was obtained from both the children and their families. Inclusion criteria were: Being between the ages of 6 and 18 years with UCP, and being levels I or II according to the Gross motor function classification system (GMFCS). Exclusion criteria included having undergone scoliosis surgery and having a rigid contracture in the spine. Ethical approval was obtained from the Clinical Research Ethics Committee of Bolu Abant izzet Baysal University (Decision number: 2023/211, date: 19.09.2023), in accordance with the Helsinki Declaration.
3.2. Measurements
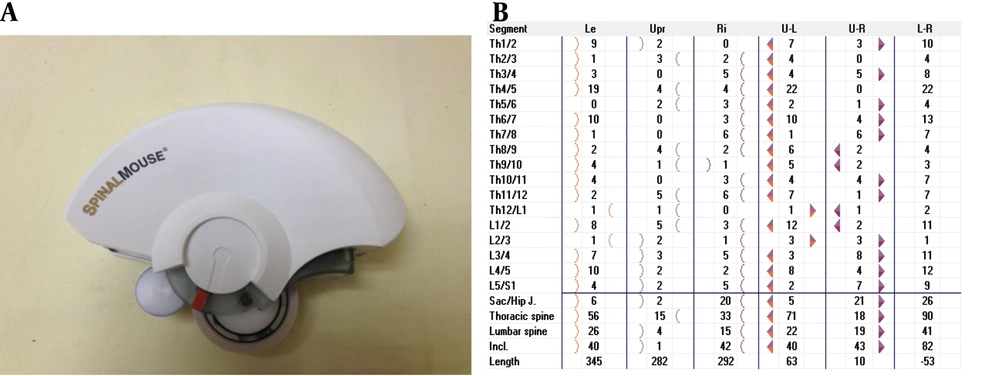
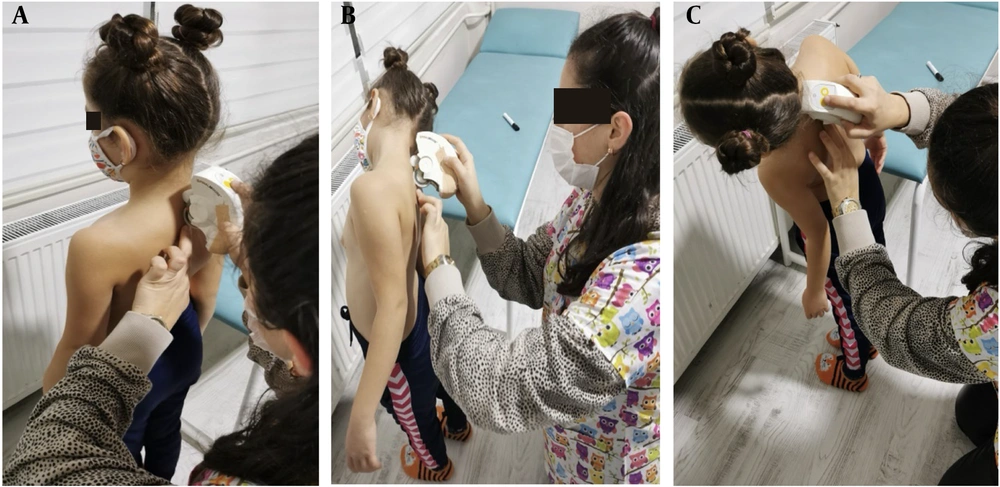
Demographic information, including age, gender, affected side, height, and body weight, was collected. Spinal posture and mobility were assessed using a computer-assisted and non-invasive electromechanical device, the Spinal Mouse (SM) (Idiag, Voletswil, Switzerland) (Figure 1). Assessments were conducted in a quiet, calm room with the assessor, child, and parent present. Measurements were performed with the SM when the child had no clothing on the trunk and was not wearing any orthoses or shoes. The assessment with the SM was performed in a standing position with the feet shoulder-width apart in the frontal plane. The SM device was moved along the spinal processes from C7 to S2 on the skin surface. Data obtained from the device was transmitted via bluetooth to a computer and recorded. Three measurements were taken in the frontal plane: Standing posture in an upright position, lateral flexion of the trunk on the affected side, and lateral flexion of the trunk on the unaffected side (Figure 2).
3.3. Statistical Analysis
Sample size calculation was performed using G-Power 3.1.9.7 software. Since no study in the literature compares SM and lateral spinal mobility in children with UCP, it was determined that at least 36 participants were needed at 90% power and a 5% error level for a t-test in dependent groups with a medium effect size of d = 0.5. It was also assumed that there would be a 20% loss of participants, and it was determined that at least 44 participants should be studied. Statistical analyses were conducted using IBM SPSS Statistics for Windows, v. 26.0 (Armonk, NY: IBM) and R Statistical Software (v4.3.0; R Core Team, 2023). Descriptive statistics were calculated as mean and standard deviation for continuous variables, and frequency and percentage for categorical variables. The Shapiro-Wilk test was used to assess the normality assumption. For numerical variables, a dependent samples t-test was performed to compare bilateral lateral spinal mobility. A significance level of P < 0.05 was considered statistically significant.
4. Results
The demographic characteristics of the 58 children with UCP who participated in this study, including age, height, body weight, Body Mass Index, gender, and affected side, are presented in Table 1. The spinal posture angles and mobility comparisons in the frontal plane, measured with the SM, are shown in Table 2. No significant difference was found between the affected and unaffected sides in terms of spinal lateral mobility angles at the sacrum-hip (P = 0.353), thoracic (P = 0.602), lumbar (P = 0.079), and inclination (P = 0.352) angles. However, a significant difference was observed in right and left lateral flexion mobility, with differences in sacrum-hip (P = 0.011) and lumbar (P = 0.000) angles.
| Variables | UCP Children (n = 58) |
|---|---|
| Age (y) | 9.4 (6 - 18) |
| Height (cm) | 134.1 (92.5 - 179) |
| Weight (kg) | 34.2 (12.5 - 105.2) |
| BMI (kg/m2) | 17.3 (11.8 - 32.87) |
| Gender | |
| Female | 30 (51.7) |
| Male | 28 (48.3) |
| Affected side | |
| Right | 36 (62.1) |
| Left | 22 (37.9) |
Demographic Characteristics of Participants with Unilateral Cerebral Palsy a
| Inclination Degree Curvatures; UP | Unilateral Cerebral Palsy Children; n = 58, Median (IQR 25/75) | ||
|---|---|---|---|
| Sacrum-hip | 4.8 (0/12) | ||
| Thorax | 8.3 (0/17) | ||
| Lumbal | 8.4 (0/20) | ||
| Total spine | 2.6 (0/13) | ||
| Mobility | UP-Affected side Latflex P; n = 58, Median (IQR 25/75) | UP-Nonaffected side Latflex P; n = 58, Median (IQR 25/75) | P-Value a |
| Sacrum-hip | 9.2 (0/37) | 8 (0/35) | 0.353 |
| Thorax | 24.2 (2/44) | 23.1 (4/59) | 0.602 |
| Lumbal | 12.2 (0/31) | 16.3 (2/36) | 0.079 |
| Total spine | 22.2 (7/42) | 21.1 (4/48) | 0.352 |
| UP | UP-Right Latflex P | UP-Left Latflex P | P-Value a |
| Sacrum-hip | 10.2 (0/37) | 7 (0/35) | 0.011 |
| Thorax | 25.6 (3/41) | 21.9 (2/59) | 0.066 |
| Lumbal | 8.1 (0/28) | 20.4 (4/36) | 0.000 |
| Total spine | 21.8 (7/34) | 21.5 (4/48) | 0.855 |
Comparison of Lateral Spinal Mobility Values in Frontal Plane
5. Discussion
In this study, which aimed to compare the spinal mobilities of the affected and unaffected sides in children with CP, it was concluded that the lateral spinal mobility angles of the affected and unaffected sides were approximately similar. It was observed that the involvement of the affected half of the trunk in CP did not negatively impact trunk lateral flexion mobility compared to the unaffected side. Studies have reported high rates of lateral curvature in children with UCP (14, 19). No difference was observed between the lateral spinal mobility angles of the trunk due to compensatory mechanisms secondary to these curvatures (16).
Postural patterns vary in children with UCP. In UCP, two primary postural patterns have been described: The pro-gravitational postural pattern (PGPP) and the anti-gravitational postural pattern (AGPP). The differences between PGPP and AGPP not only involve characteristic weight-bearing on the unaffected or affected side of the body but also include significant differences in the orientation of the spine, pelvis, and shoulder girdle. In children with AGPP, the pelvis exhibits upward obliquity on the affected side, whereas in children with PGPP, the pelvis shows downward obliquity. Lateral spinal curvature has been reported in the majority of the children examined (84%). In all children with AGPP, convexity of the spine is observed toward the unaffected side, while in children with PGPP, the convexity is directed toward the affected side. More importantly, in children with AGPP, the affected side bears less weight, whereas in children with PGPP, the affected side bears more weight. The characteristic postural pathology in all children with CP should be addressed, as it affects overall functional efficiency (19).
The postural patterns of the children included in our study were not assessed. The similarity between the lateral spinal mobility angles of the affected and unaffected sides in this study may be attributed to the postural patterns specific to CP. One of the findings of our study was that the sacrum-hip angles were greater on the right side. The majority of the children in this study (62.1%) had right-sided involvement. In the right lateral flexion position, which is the affected side for most children, sacrum-hip and thoracic angles were greater, while in the left lateral flexion position, lumbar angles were greater. We hypothesize that the increased lumbar mobility on the left side may serve as a compensatory mechanism. This result is based on the compensatory mechanisms that occur in the trunk as a result of postural deformities to keep the center of gravity within the support surface and maintain balance (16).
Scoliosis is a deformity that negatively affects spinal alignment and mobility. In scoliosis, a smaller lateral curvature angle in the frontal plane indicates higher frontal spinal mobility (20). Porsnok et al. assessed only the presence of scoliosis and scoliosis angles in the frontal plane in their study on spinal alignment in children with UCP. No evaluation was made regarding lateral spinal mobility. Based on these findings, scoliosis was reported in 40% of children and they were found to be at risk for developing spinal deformities (14). In this study, the presence of scoliosis in children with UCP was not assessed. Scoliosis is considered a factor that affects lateral spinal mobility (20). We hypothesize that any scoliosis present in the children may have influenced the lateral spinal mobility angles.
There are very few studies in the literature that have assessed spinal alignment and mobility in children with UCP (6, 14). Spinal angulations in a study in which spinal posture and mobility of children with UCP were evaluated in the frontal plane with a SM were similar to the angle values in our study. However, in these studies, the lateral spinal mobility of the affected and unaffected sides in children with UCP has not been compared (6). Therefore, we are unable to relate the results of our study to those of other studies. Suh et al. reported differences in thoracolumbar kyphosis, lumbar lordosis, pelvic tilt, and sacral slope angles in children with CP compared to typically developing children in their study in which they examined spinopelvic mobility in the sagittal plane by radiography (21). In the literature, studies examining spinal posture and mobility in the frontal plane in individuals with CP emphasized scoliosis and included interventions for scoliosis (13, 16). In studies conducted in the sagittal plane, the relationship between spinal angulations was emphasized. Accordingly, it was emphasized that there was a relationship between sacral inclination and lumbar lordosis and between lumbar lordosis and thoracic kyphosis (22).
It has been reported that frontal curvature angles are higher in children with UCP compared to their peers (14). In this study, the lateral spinal curvatures and lateral spinal mobility angles of the affected and unaffected sides were found to be similar. We hypothesize that this may be due to the involvement of not only the affected half of the body in children with UCP but also the "unaffected" half, which we typically consider as intact. In UCP, sensorimotor integration, bimanual coordination, and motor planning impairments affect both sides of the body (23). In the side where more severe sensory impairments are observed, the kinematics of the upper extremity may be affected, which in turn can influence spinal alignment and mobility (24). It has been suggested that the dominant side in UCP should be considered as the less affected side rather than the unaffected side (25).
There are several limitations in our study. The first limitation is that spinal mobility was assessed only in the frontal plane. The rotational movements of the spine on both sides of the body could not be evaluated. The SM provides information only on spinal angles and mobility in the sagittal and frontal planes (6). The second limitation is that lower extremity anthropometric measurements, which could influence spinal alignment, were not taken in this study. Also, postural patterns specific to the children were not assessed. In future studies, the existing postural patterns of children with UCP could be identified, and spinal alignment and mobility could be evaluated and compared according to PGPP and AGPP patterns.
5.1. Conclusions
The lateral spinal mobility angles of the affected and unaffected sides in children with UCP are similar. We attribute these results to the fact that UCP is a condition that affects the entire body. Somatosensation is a parameter that influences functionality and posture in children with UCP. Including the somatosensory system in the assessment protocols of these children may help identify the underlying causes of postural disorders (26). In the rehabilitation of children with CP, healthcare professionals should focus on developing: (A) Proper spinal curvature (kyphosis or lordosis) and a neutral pelvic position in the sagittal plane, as well as the symmetry of pelvic, trunk, and shoulder girdle orientation in the coronal plane; (B) motor control of pelvic rotation, hip abduction, knee flexion, and ankle dorsiflexion (27). Additionally, attention should be paid to preventing the development of asymmetric posture during maturation (7).