1. Background
Troponins (Tp) are cardiac biomarkers used to indicate myocardial injury. Troponin I was first defined in 1987 as a specific biomarker for myocardial infarction (MI). Troponins are complex proteins found in both skeletal and cardiac muscle fibers. There are three different forms: Troponin C (TnC), cardiac troponin T (cTnT), and cardiac troponin I (cTnI). Only cTnI and cTnT are found in the heart (1).
Recent studies suggest that exercise can lead to myocardial damage and can cause elevations in serum cTnT and cTnI levels (2). It has also been shown that, after exercise, there may be an increase in serum cTnI levels without myocardial damage in healthy participants. An increase in cardiac biomarkers after exercise has been demonstrated in half-marathon, full-marathon, ultra-marathon, triathlon, and bicycle racers (3, 4). The range of exercise-induced serum cardiac Tp elevation has been reported to vary from 0% to 78% (5, 6).
It is thought that the exercise-induced cTnI response is due to the leakage of cardiac Tps from the cytoplasm with physical activity, whereas the increase in cardiac Tp following myocardial ischemia and infarction is due to the loss of cellular integrity (7).
Pediatric patients are of particular interest in the context of exercise-induced Tp elevations because their cardiovascular systems are still developing, and their response to physical stress may differ from that of adults (8). Differentiating physiological biomarker elevations from pathological conditions is critical to avoid unnecessary anxiety, testing, and interventions in this age group (9-11).
While exercise-induced increases in serum cardiac biomarkers are well-documented in adults (12), limited data exist regarding such changes in children and adolescents (13). This study seeks to fill this gap by exploring the relationship between exercise and cTnI levels in pediatric patients, contributing new insights to the growing body of literature.
2. Objectives
The primary aim of this study was to investigate the relationship between serum cTnI elevations and exercise, and to evaluate the association of this increase with age, gender, type, and duration of exercise. Additionally, the study aimed to demonstrate that not all elevations in serum cTnI levels in children are related to MI; after exercise, serum cTnI levels can increase as a physiological response. Furthermore, we believe that emergency physicians should consider exercise-induced Tp elevation in pediatric patients presenting to the emergency department with musculoskeletal pain and/or other non-cardiac complaints following exercise. This study was conducted to raise awareness among physicians about exercise-related Tp elevation.
3. Methods
3.1. Selection and Identification of Cases
This retrospective study was conducted at the Pediatric Emergency Clinic of Prof. Dr. Cemil Taşcıoğlu City Hospital in Istanbul, Turkey, between January 1, 2023, and December 31, 2023. Pediatric participants who visited the Pediatric Emergency Clinic within 24 hours of experiencing chest pain following exercise and had elevated serum cTnI levels were included. A total of 25 participants who presented to our hospital with chest pain after exercise and had serum cTnI levels > 14 ng/L were enrolled in the study over a 12-month period.
Participants with chronic cardiac conditions or normal serum cTnI levels were excluded. Those presenting with clinical signs or laboratory parameters indicative of infection were also excluded. Additionally, participants older than 18 years were not included in the study.
Electronic medical records, laboratory information systems, and structured follow-up telephone interviews with patients and/or caregivers were used as data sources. To ensure the accurate extraction of exercise type, duration, and timing in relation to the onset of symptoms, two independent physicians reviewed the clinical records. Any conflicts were resolved through consensus.
At our hospital, additional tests, including echocardiography (ECHO), are routinely performed for pediatric patients with elevated Tp levels. Patients with no pathological findings on ECHO are scheduled for follow-up at 24 and 48 hours, during which Tp levels are monitored. This study reviewed patients who were followed in this manner at our hospital over a one-year period.
Patients with a history of physical activity or sports were included in the study, while those who could not be contacted for follow-up Tp monitoring on the second and third days were excluded. Contact information was obtained from patient records, and inquiries about their sports history were made. Sports history details were recorded based on the date of their initial visit. The duration and type of exercise were quantified through structured patient interviews, which were conducted during follow-up visits. Participants were asked to specify the exact sport (e.g., football, basketball), the total duration of each session, and how frequently they participated in these activities per week. Exercise intensity was not directly measured but was inferred from the sport category and self-reported performance.
The exclusion criteria for this study were carefully defined to ensure the accuracy and significance of the results. Participants were excluded if they had any known familial or acquired cardiac conditions, including structural cardiovascular abnormalities, cardiomyopathies, or arrhythmias. Individuals with systemic disorders that might influence the cardiovascular system were also excluded, as these conditions could independently affect cardiac biomarker levels. Furthermore, participants exhibiting clinical or laboratory indicators of active infection, such as fever or elevated C-reactive protein (CRP) and white blood cell (WBC) counts, were excluded from the study. Participants with normal baseline serum cTnI levels, defined as ≤ 14 ng/L, were eliminated to focus the analysis on individuals showing post-exercise biomarker elevations. Patients with whom contact could not be established were also excluded from the study.
3.2. Technical Information
This is a retrospective study conducted at Prof. Dr. Cemil Taşcıoğlu City Hospital. By reviewing one year of data from our hospital, the records of patients who presented to the pediatric emergency department with chest pain developing after sports activities were obtained. Among these patients, those with serum cTnI levels above the reference range were included in the study. At our hospital, the reference value for a normal serum cTnI level is defined as below 14 ng/L. Participants with serum cTnI levels higher than 14 ng/L were included in the study.
The patient files were analyzed retrospectively. The age, gender, height, weight of the participants, and the type and duration of the sport they practiced were recorded. Serum cTnI levels at the time of first admission, after 12 hours, on the second day, and on the third day were documented. Creatine kinase-myocardial band (CK-MB) levels, measured on the day of exercise, on the second day, and on the third day, were also recorded. In addition, biochemical blood parameters of the participants were extracted from the patient files. Resting electrocardiograms (ECG) and ECHO results were evaluated.
Ethical approval for the study was obtained from the Clinical Research Ethics Committee of Prof. Dr. Cemil Taşcıoğlu City Hospital (ethics committee file number: 48670771-514.99-221099101).
3.3. Statistical Analysis
Statistical analyses were conducted using the IBM statistical package for the social sciences (SPSS) for Windows, version 22.0. The normality distribution of variables was tested using the Shapiro-Wilk test. The t-test and Pearson correlation analysis were employed for normally distributed variables, while the Mann-Whitney U test and Spearman’s Rho correlation analysis were utilized for non-normally distributed variables. Results with a P-value of less than 0.05 were considered statistically significant. Findings were presented as mean ± standard deviation or median (25th - 75th percentiles).
To address potential confounding, multivariable regression models were initially planned; however, due to the limited sample size, adjustments were constrained. Therefore, partial correlation analysis was used to control for age when evaluating the association between cTnI levels and duration of exercise.
4. Results
A total of 25 participants were included in the study. Nineteen of the participants were male. The mean age of the participants was 13.32 ± 3.45 years. None of the participants reported any cardiac symptoms following exercise.
The blood pressure (BP) of all participants included in this study was considered normal for their age. The mean systolic BP was 103.2 ± 12.23 mmHg, and the mean diastolic BP was 70.0 ± 7.35 mmHg. The mean heart rate of the participants was 83.4 beats per minute.
Only one participant exhibited ECG abnormalities (sinus bradycardia), with no correlation found between cTnI elevation and ECG findings in this case. All other ECG results were unremarkable. The ECHO results were found to be normal in all participants. The mean left ventricular (LV) internal diameter in diastole (LVIDd) was 47.88 ± 5.85 mm, and the mean left ventricular internal diameter in systole (LVIDs) was 28.32 ± 4.70 mm.
The mean cTnI value obtained at the first admission was 133.03 ± 129.46 ng/L. During follow-up, the mean cTnI levels decreased to 78.05 ± 97.16 ng/L in 12 hours, and 16.01 ± 18.42 ng/L in 24 hours, and 3.25 ± 2.48 ng/L at 48 hours.
At first admission, the mean CK-MB level was 16.06 ± 12.11 ng/L; this decreased to 13.17 ± 10.72 ng/L in 24 hours and to 4.00 ± 4.88 ng/L in 48 hours.
Participants presented to the hospital after playing football and basketball. Although participants primarily engaged in football (n = 15) and basketball (n = 10), no statistically significant difference in cTnI levels was observed between the two groups (P > 0.05). However, the small sample size limits the power of this comparison.
The mean duration of exercise was 1.88 ± 0.61 hours. When participants were asked when they first started engaging in sports activities, the mean duration was 2.00 ± 2.21 years ago.
All participants' biochemical blood parameters were within the normal range. Demographic, clinical findings, and laboratory test results of the participants are shown in Table 1.
| Parameters | Mean ± SD |
|---|---|
| Weight (kg) | 56.08 ± 16.12 |
| Height (cm) | 1.61 ± 0.18 |
| Heart rate (bpm) | 83.40 ± 5.45 |
| Systolic BP (mmHg) | 103.20 ± 12.23 |
| Diastolic BP (mmHg) | 70.00 ± 7.35 |
| CK (IU/L) | 201.90 ± 159.86 |
| CK-MB 1 (ng/mL) | 16.06 ± 12.11 |
| CK-MB 2 (ng/mL) | 13.17 ± 10.72 |
| CK-MB 3 (ng/mL) | 4.00 ± 4.88 |
| Tp I 1 (ng/L) | 133.03 ± 129.46 |
| Tp I 2 (ng/L) | 78.05 ± 97.16 |
| Tp I 3 (ng/L) | 16.0 ± 18.42 |
| Tp I 4 (ng/L) | 3.25 ± 2.48 |
| Total cholesterol (mg/dL) | 167.05 ± 25.62 |
| Triglyceride (mg/dL) | 107.10 ± 93.32 |
| LDL cholesterol (mg/dL) | 90.52 ± 18.99 |
| HDL cholesterol (mg/dL) | 55.21 ± 10.91 |
| Glucose (mg/dL) | 88.92 ± 18.23 |
| AST (IU/L) | 29.40 ± 17.33 |
| ALT (IU/L) | 15.84 ± 6.89 |
| LDH (IU/L) | 232.60 ± 45.03 |
| Urea (mg/dL) | 30.91 ± 5.55 |
| Creatinine (mg/dL) | 0.58 ± 0.15 |
| Time of exercise (h) | 1.88 ± 0.61 |
| Time of exercise (y) | 2.00 ± 2.21 |
Abbreviations: CK, creatine-kinase; CK-MB, MB isoenzyme of creatine-kinase; LDL cholesterol, low-density lipoprotein cholesterol; HDL cholesterol, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Tp, troponin; BP, blood pressure.
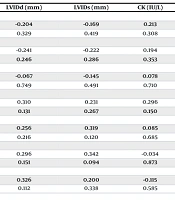
In the present study, there was a negative correlation between cTnI levels and the longevity of participants' exercise history (P = 0.024; r = -0.450). Additionally, there was a negative correlation between age and cTnI levels (P = 0.001; r = -0.620). Furthermore, a significant positive correlation was found between post-exercise cTnI levels and the duration of exercise (P = 0.000; r = 0.924). There was no significant correlation between cTnI, CK-MB levels, and other biochemical markers (Table 2).
| Parameters | Time of Exercise (h) | Time of Exercise (y) | LVIDd (mm) | LVIDs (mm) | CK (IU/L) | Age (y) | Fractional Shortening (%) |
|---|---|---|---|---|---|---|---|
| CK-MB 1 (ng/mL) | |||||||
| r | -0.314 | -0.002 | -0.204 | -0.169 | 0.213 | -0.284 | -0.112 |
| P | 0.126 | 0.992 | 0.329 | 0.419 | 0.308 | 0.169 | 0.595 |
| CK-MB 2 (ng/mL) | |||||||
| r | -0.372 | 0.153 | -0.241 | -0.222 | 0.194 | -0.387 | -0.046 |
| P | 0.067 | 0.466 | 0.246 | 0.286 | 0.353 | 0.56 | 0.829 |
| CK-MB 3 (ng/mL) | |||||||
| r | -0.145 | 0.084 | -0.067 | -0.145 | 0.078 | -0.177 | 0.191 |
| P | 0.488 | 0.689 | 0.749 | 0.491 | 0.710 | 0.397 | 0.361 |
| Tp I 1 (ng/L) | |||||||
| r | 0.924 | -0.450 | 0.310 | 0.231 | 0.296 | 0.620 | 0.076 |
| P | 0.000 | 0.024 | 0.131 | 0.267 | 0.150 | 0.001 | 0.719 |
| Tp I 2 (ng/L) | |||||||
| r | 0.902 | -0.454 | 0.256 | 0.319 | 0.085 | 0.672 | 0.005 |
| P | 0.000 | 0.023 | 0.216 | 0.120 | 0.685 | 0.000 | 0.981 |
| Tp I 3 (ng/L) | |||||||
| r | 0.523 | -0.318 | 0.296 | 0.342 | -0.034 | 0.411 | -0.096 |
| P | 0.007 | 0.121 | 0.151 | 0.094 | 0.873 | 0.041 | 0.647 |
| Tp I 4 (ng/L) | |||||||
| r | 0.589 | -0.196 | 0.326 | 0.200 | -0.115 | 0.585 | 0.128 |
| P | 0.002 | 0.348 | 0.112 | 0.338 | 0.585 | 0.002 | 0.543 |
Abbreviations: CK, creatine-kinase; CK-MB, MB isoenzyme of creatine-kinase; LVIDd, left ventricular internal diameter end diastole; LVIDs, left ventricular internal diameter end systole; Tp, troponin.
5. Discussion
The objective of this study is to compare pre-exercise and post-exercise levels of serum cardiac biomarkers. Furthermore, it aims to analyze the rates of exercise-induced elevations according to age, gender, exercise intensity, and duration in children. There are few studies examining cardiac biomarker responses to exercise in children and adolescents. Additionally, these studies often had small sample sizes and unequal exercise exposure, limiting their statistical power.
The data collected in this study with 25 participants suggest that serum cTnI elevation may occur after exercises such as football and basketball in the pediatric population. This response, however, is not considered a pathological occurrence. Previous studies utilizing a measurement design before and after exercise suggest that concentrations of serum cTnI and cTnT are elevated in more than half of athletes after exercising (3). Moreover, it has been noted that young athletes who participate in activities such as marathon running also exhibit elevated serum cTnT and cTnI levels (9). Additionally, Papamichail et al. showed that physical activities can lead to the release of serum cTnI in children and adolescents (10).
However, there is insufficient direct evidence to either support or dismiss the notion of a singular mechanism responsible for the rise in serum cTnI concentrations after exercise. Several potential mechanisms may underlie exercise-induced cTnI elevations in the pediatric population. These include increased myocardial wall stress, transient cardiomyocyte membrane permeability, and heightened mechanical workload during exercise. Unlike MI, these processes do not involve irreversible myocyte necrosis.
Recent studies have suggested that the heart plays a principal role in meeting the increased total body oxygen demand during physical activity. This is achieved through an increase in both heart rate and stroke volume. Consequently, there is a rise in myocardial oxygen demand, coronary blood flow, and cardiac preload and afterload. These adaptive reactions may trigger an increase in cardiomyocyte distress, potentially leading to the passive diffusion of cTn from the cell into the extracellular environment (11).
Several studies have demonstrated that the release of cTnI from the myocyte membrane following physical activity is caused by increased cell membrane permeability, rather than myocyte necrosis, and is considered a harmless event (3, 7).
Studies show that the increase and rapid decrease in cTnI levels after sports are temporary, resulting from a reversible cellular process and representing a physiological response to physical activity (14, 15). Our findings are consistent with other studies showing that maximum serum cTnI concentrations occur three to five hours after exercise. Additionally, in our study, it was observed that elevated levels decreased to normal within 24 - 48 hours. These results are in accordance with data gathered from studies involving adults (10).
In our study, we found that serum cTnI levels were higher in males after exercise compared to females. However, other studies in the literature have shown that gender has little impact on this increase, possibly due to the protective role of estrogen against cTnI elevation (16, 17).
Our findings suggest that there was a negative correlation between age and post-exercise serum cTnI levels. Similarly, in a study conducted with 766 runners, which included both pediatric and adult participants, it was observed that younger participants had higher serum cTnI levels after a marathon (18). It has been hypothesized that the myocardial tissue of younger athletes may be more prone to exercise-induced elevations in serum cTnI levels compared to older athletes. This phenomenon may be due to the fact that the myocardium is less mature in younger individuals. Considering the moderate cardiovascular risk among children and adolescents, it is crucial to carefully examine cardiac biomarker levels. It is important to recognize that the immature myocardium of pediatric participants is more vulnerable to injury compared to that of adult athletes (19).
However, one study suggests that there is no significant correlation between serum cTnI release and pubertal status (10). This suggests that, in order to clarify the relationship between age and serum cTnI levels, larger studies should be conducted in the future, including both children and adolescents, to better understand exercise-induced cTnI release.
Although the effects of sport type, age, and gender on post-exercise serum cTnI levels have not been fully defined, existing data indicate that the intensity and duration of sports have significant impacts on the elevation of cardiac enzymes after exercise. While the literature mentions that exercise duration is associated with changes in both cTnI and NT-proBNP levels, it also emphasizes that exercise intensity is a major determinant of cTnI release. Changes in cTnI are believed to be influenced by the initial cTnI concentration, exercise intensity, and exercise duration.
A recent study revealed that the response of individuals' cTnI levels, influenced by the duration of high-intensity exercise and the timing of cTnI sampling, contributes significantly to the variance observed in exercise-induced serum cTnI elevations. Troponin levels are initially lower and then peak at around 3 hours and 24 hours post-exercise, respectively. The most significant differences in serum cTnI levels are observed 3 hours after the most strenuous activities compared to the least demanding exercises. Notably, no variations were observed in baseline ECHO parameters or race performance among participants with the highest or lowest levels of cTnI (20).
In our study, we found a significant correlation between the consecutive cTnI levels obtained from participants who presented to our hospital after playing football and basketball and the duration of exercise. The athletes reported their exercise durations, and it was observed that those who exercised for two hours or longer had higher cTnI values than other athletes. Blood pressure, ECG, and ECHO were performed on these participants four hours after exercise, and no cardiac pathology was observed. Similarly, there are studies in the literature that show a significant association between longer exercise duration and higher levels of serum cTnI. Both the duration and intensity of exercise were associated with serum cTnI and NT-proBNP concentrations (20).
In our study, we also found a negative correlation between the lengths of time participants had been engaged in sports and their cTnI levels. We observed that those who had been participating in sports for longer periods and on a regular basis had higher Z-scores for LV end-diastolic diameters in M-mode, as measured by ECHO, and lower heart rates. Interestingly, serum cTnI levels were lower in individuals who regularly engaged in sports for longer periods, possibly due to the fact that their heart rates did not increase significantly during physical activity and their myocardial contraction force was lower. A previous study showed higher serum cTnI levels in less experienced runners, suggesting that the body may adapt to repeated physical activity by modulating cTnI release (20).
Another study, conducted with young amateur athletes participating in a 12-week exercise program, observed a positive correlation between systolic BP and serum cTnI levels. It also demonstrated an association between cTnI levels and mean arterial pressure. Furthermore, a significant association was found between cTnI levels and resting heart rate (21). These findings suggest that there may be an adaptive mechanism to repeated physical activity. With larger study groups, this mechanism could be better understood.
The use of CK-MB for the diagnosis and management of acute coronary syndromes was once widely accepted; however, it was later demonstrated to lack cardiac tissue specificity and sensitivity. In our study, CK-MB levels were not significantly elevated after exercise. However, Rahnama et al. (22) found high CK-MB levels after football playing, which may result from muscle injuries sustained during football games. Additionally, another study showed that among athletes, skeletal muscle CK-MB concentrations were higher and released in response to exercise-induced muscle injury (23). Expanding the size of our study group could yield more substantial results regarding this issue.
All participants included in our study were evaluated with ECHO. No pathological findings were observed in the ECHO results of any of the participants. Most studies investigating the association between serum cTnI levels and exercise have been designed for adult populations. In one such study, reductions in LV ejection fraction and diastolic function were found on ECHO in marathon runners (23). However, findings from most clinical studies have shown no association between cTnI concentrations and coronary artery disease (24).
There was no significant correlation between cTnI levels and other biochemical parameters in our study.
As a result, serum cTnI elevation after exercise is generally regarded as a physiological response and a benign phenomenon. However, studies have been conducted to explore the association between exercise-induced serum cTnI release and both cardiovascular events and the prevalence of previously undiagnosed obstructive coronary artery disease in adult participants. More research is necessary to uncover the underlying mechanisms behind the exercise-induced release of serum cTnI and to establish whether post-exercise serum cTnI levels could serve as a novel indicator of cardiovascular risk.
Clinically, the results of our study suggest that elevated cTnI levels in pediatric patients presenting with chest pain following exercise should not be immediately interpreted as pathological. In the absence of ECG or echocardiographic abnormalities, these elevations may reflect a benign, transient response to physical exertion. Healthcare providers should carefully consider recent physical activity and exercise history when evaluating such cases.
It is important to note that the results of our study should be interpreted within the context of its limitations. Specifically, the limitations include the predominance of male participants and the relatively small sample size.
5.1. Conclusions
Our research has indicated that physical activity is associated with the release of serum cTnI in children and adolescents, which is a benign phenomenon. In clinical practice, when we encounter cases with elevated serum cTnI levels, we typically consider cardiac pathologies. However, it is important to recognize that elevated cardiac enzymes may represent a benign response to exercise in children and adolescents.
Therefore, we would like to emphasize that exercise history should be carefully assessed when elevated Tp I levels are detected in pediatric patients. This consideration may help avoid unnecessary diagnostic procedures and alleviate concern in cases where elevated cTnI is a physiological, rather than pathological, response.
In general, it should be remembered that serum cTnI levels may physiologically increase after exercise. More studies in this area, along with data obtained from the long-term follow-up of these participants, will contribute valuable insights to the literature.
5.2. Limitations
This study has several limitations. The generalizability of the findings is restricted by the male predominance (76%) and the small sample size. Its retrospective design limits the accuracy of the data, particularly concerning self-reported exercise intensity and duration. Without pre-exercise cTnI measurements, we are unable to definitively attribute the observed elevations solely to exercise. The absence of a sedentary control group further impedes interpretation, and the focus on football and basketball limits the applicability of the findings to other types of exercise.
Long-term effects were not evaluated due to the 48-hour follow-up period. Additionally, we were unable to account for potential confounders such as hydration status, Body Mass Index (BMI), pubertal stage, or subclinical illness, and we lacked data on clinical outcomes. Although some of these issues were previously acknowledged, we now emphasize their implications and recommend strategies for future research, such as conducting longitudinal studies to evaluate the long-term effects of repeated post-exercise cTnI elevations.