1. Background
Extramedullary relapse (EMR) is a recurrence of leukemia in sites other than the bone marrow, and it exhibits a relatively rare presentation of relapse of acute leukemia (1). However, the exact incidence of EMR remains unclear because the reported incidence has varied in previous studies. About 3% - 5% of patients are estimated to have an EMR in acute myeloid leukemia (AML) at some point in their lives (2-4). In acute lymphoid leukemia (ALL), approximately 20% will experience relapse. The cumulative risk of relapse is 11% in a 10- year period, including EMR and bone marrow relapse (BMR) (5-7). EMR remains an important cause of treatment failure among patients with acute leukemia (3). The prognosis of EMR is poor with a median survival of only a few months (3, 4). Early detection of these relapses may improve the prognosis (1). The clinical characteristics and radiologic findings of EMR are limited because of its low incidence (1, 8). However, EMR cases have been increasingly reported in the last few years (9).
2. Objectives
We aimed to describe the demographic and clinical features, radiologic findings, and clinical outcomes for children diagnosed with EMR in acute leukemia.
3. Patients and Methods
Our institutional review board approved this retrospective study and waived the requirement for an informed consent. We used our computerized hospital information system to identify all children under 16 years old who were diagnosed with EMR with acute leukemia presenting from January 1, 2009 to December 31, 2011 at our hospital. We respectively reviewed the clinical records and radiologic studies of these patients.
The diagnosis of EMR was established by tissue biopsy in six cases, examination of the CSF in seven cases, and clinical course and radiologic findings in residual cases. All patients who presented with EMR underwent a BM biopsy and aspiration for the evaluation of a possible coincident BMR within four (1 - 7) days after EMR diagnosis. Patients’ past medical records were also reviewed for age, gender, white blood cell count (WBC), French-American-British (FAB) morphology, cytogenetics, and the presence of extramedullary disease (EMD) at initial diagnosis of acute leukemia. Time to and site of EMR, radiologic findings, and clinical course were also reviewed.
4. Results
4.1. Patient Characteristics
The number of EMR among acute leukemia patients was 22 ((M: F = 14:8; mean age, 7.30 (2.1 - 15.7) years and initial diagnosis of acute leukemia, 1.7 - 15 years)). Among the patients, 14 had ALL and 8 patients had AML. Median WBC initially diagnosed as acute leukemia was 80.0 × 109/L (range 3 - 475.5 × 109/L). Among the 22 patients, 14 had FAB L1 ALL, 5 had FAB M2 AML, and each of the 3 remaining had FAB M0, M4, and M7, respectively. Cytogenetic results were available for 16 of the 22 patients. Leukemic cells of four patients had a normal karyotype, five had t(8: 21), two had t(9: 22), and one each had t(1: 18), t(17: 19), t(5: 10), t(2: 12), and t(1: 17), respectively. Only one of the patients had EMD at the first diagnosis (site of EMD: bilateral ocular/temporalis muscles, paranasal sinuses, skull base, nasal cavity, and both mastoid spaces).
EMR occurred with a median of 30.2 (4.4 - 117.5) months from the initial diagnosis of acute leukemia and 22.7 (2 - 116.5) months from complete remission (CR).
The disease-related features of the 22 patients with EMR are summarized in Table 1.
| Case | Age | Sex | Type | FAB | WBC (×109/L) | Cytogenetics | Extramedullary Disease |
|---|---|---|---|---|---|---|---|
| 1.7 | M | AM | M7 | N/A | 46,XY | No | |
| 8 | F | L | M2 | 21.75 | t(8, 21) | No | |
| 11 | M | AM | M0 | 65.80 | t(5, 10) | No | |
| 11 | F | L | M2 | 40.46 | t(8, 21) | No | |
| 15 | M | AM | M2 | 3.01 | t(8, 21) | No | |
| 9 | M | L | M2 | 8.25 | t(8, 21) | No | |
| 12 | M | AM | M4 | 23.28 | 46, XY | No | |
| 2 | M | L | M2 | 24.06 | t(8, 21) | No | |
| 10 | F | AM | L1 | 67.21 | t(17, 19) | Yes | |
| 4 | M | L | L1 | 5.0 | N/A | No | |
| 2.1 | F | AM | L1 | 47.99 | t(2, 12) | No | |
| 12 | M | L | L1 | N/A | N/A | No | |
| 13 | M | AM | L1 | N/A | N/A | No | |
| 6 | M | L | L1 | 11.66 | t(1, 18) | No | |
| 2 | F | AM | L1 | N/A | N/A | No | |
| 7 | F | L | L1 | 475.52 | t(9, 22) | No | |
| 6 | M | ALL | L1 | 88.35 | t(9, 22) | No | |
| 15 | M | ALL | L1 | 66.0 | 46, XY | No | |
| 4 | F | ALL | L1 | 7.4 | N/A | No | |
| 7 | F | ALL | L1 | 290.82 | t(1, 17) | No | |
| 3 | M | ALL | L1 | 69.85 | 46, XY | No | |
| 3 | M | ALL | L1 | N/A | N/A | No |
Abbreviation: N/A, not available.
abilateral ocular/temporalis muscles, paranasal sinuses, skull base, nasal cavity, and both mastoid spaces
4.2. Sites of EMR
Table 2 summarizes the site distribution of the EMR. The site of EMR included the following: CSF (n = 7), brain (n = 2), brachial and sacral plexus (n = 2), bone (n = 6), kidney (n = 4), muscle (n = 2), liver (n = 2), lung (n = 1), pleura (n = 1), pancreas (n = 1), intestine (n = 1), peritoneum and omentum (n = 1), parotid gland (n = 1), lymph node (n = 2), breast (n = 1), ovary and uterus (n = 1), skin (n = 1), and testis (n = 2). The central nervous system (CNS), bone, and kidney were the most common sites of EMR. Among the 22 patients, a single site of EMR was found in 13 patients and multiple sites in 9 patients. For the 5 patients with bone involvement, sites included the mastoid and temporal bone, sacrum, ulna, radius, and humerus. The sites of EMR were predominantly the CSF and kidney in ALL (29.2% and 16.7%, respectively) and the CNS except for CSF and bone in AML (28.6% and 42.8%, respectively).
| Sites of Extramedullary Relapse | AML | ALL | Total |
|---|---|---|---|
| 4 | 7 | 11 | |
| Brain | 2 | 0 | 2 |
| Brachial and sacral plexus | 2 | 0 | 2 |
| CSF | 0 | 7 | 7 |
| 6 | 0 | 6 | |
| 0 | 4 | 4 | |
| 2 | 0 | 2 | |
| 0 | 2 | 2 | |
| 1 | 0 | 1 | |
| 1 | 0 | 1 | |
| 0 | 1 | 1 | |
| 0 | 1 | 1 | |
| 0 | 1 | 1 | |
| 0 | 1 | 1 | |
| 0 | 2 | 2 | |
| 0 | 1 | 1 | |
| 0 | 1 | 1 | |
| 0 | 2 | 2 | |
| 0 | 1 | 1 | |
| 14 | 24 | 38 |
4.3. Clinical Characteristics Correlated With EMR
Clinical symptoms included soft tissue or scrotal swelling (n = 5), pain (n = 6), fever (n = 4), headache with vomiting (n = 2), and no symptoms (n = 5). Among the seven patients with CSF relapse, five patients had no significant symptoms with the detection of CSF relapse during a routine follow-up. By contrast, for the patients with other EMR, all patients had symptoms, such as swelling, pain, and fever. Only one case of EMR with massive pancreatic involvement showed abnormality in blood chemistry that had led to it being mistaken for pancreatitis.
Among the 11 patients with symptoms of swelling and pain that involved the area, 4 patients (36.4%) had additional relapsed sites without presenting symptoms.
At the time of EMR, examination of the BM aspirate did not show any evidence of leukemic recurrence in 10 (45.5%) patients. Seven (31.8%) patients had BMR at the time of EMR and five (22.7%) patients had it 1 - 7.2 months (median, 3.4 months) later (Table 3).
| Case | Type | EMR Sites | Treatment | BMR | Treatment Response | CR of BMR | CR of EMR |
|---|---|---|---|---|---|---|---|
| AML | Bone, brain | CTx | Yes | Expire | - | - | |
| AML | Pleura, bone, muscle | CTx | Yes | Expire | - | - | |
| AML | Brachial plexus | CTx | No | CR | - | 56 weeks | |
| AML | Bone, sacral plexus | CTx | No | CR | - | 4 weeks | |
| AML | Muscles, bone | CTx | No | CR | - | 12 weeks | |
| AML | Brain | CTx | Yes | FU loss | - | - | |
| AML | Lung, bone | CTx | Yes | Expire | - | - | |
| AML | Bone | CTx | No | CR | - | 6.3 weeks | |
| ALL | Skin, LN, ovary, uterus, breast, liver, kidney | CTx | 23 weeks | CR | 4 weeks | 5.4 weeks | |
| ALL | Testis | CTx, RTx | No | CR | - | 10.2 weeks | |
| ALL | Parotid gland, LN, liver, kidney | CTx | 24 weeks | CR | 13.2 weeks | 2.6 weeks | |
| ALL | Testis | CTx | No | Expire | - | - | |
| ALL | Kidney | CTx | 4 weeks | Expire | - | - | |
| ALL | Pancreas, kidney, peritoneum, omentum | CTx | 24 weeks | FU loss | - | - | |
| ALL | Intestine | CTx | No | CR | - | 4.2 weeks | |
| ALL | CSF | CTx | No | CR | - | 3 weeks | |
| ALL | CSF | CTx | Yes | CR | 16 weeks | 4.2 weeks | |
| ALL | CSF | CTx | No | CR | - | 11.6 weeks | |
| ALL | CSF | CTx | 20 weeks | CR | 20 weeks | 42 weeks | |
| ALL | CSF | CTx | No | CR | - | 5.4 weeks | |
| ALL | CSF | CTx | Yes | CR | 6.3 weeks | 6.5 weeks | |
| ALL | CSF | CTx | Yes | CR | 2 weeks | 5.4 weeks |
4.4. Radiologic Features of EMR
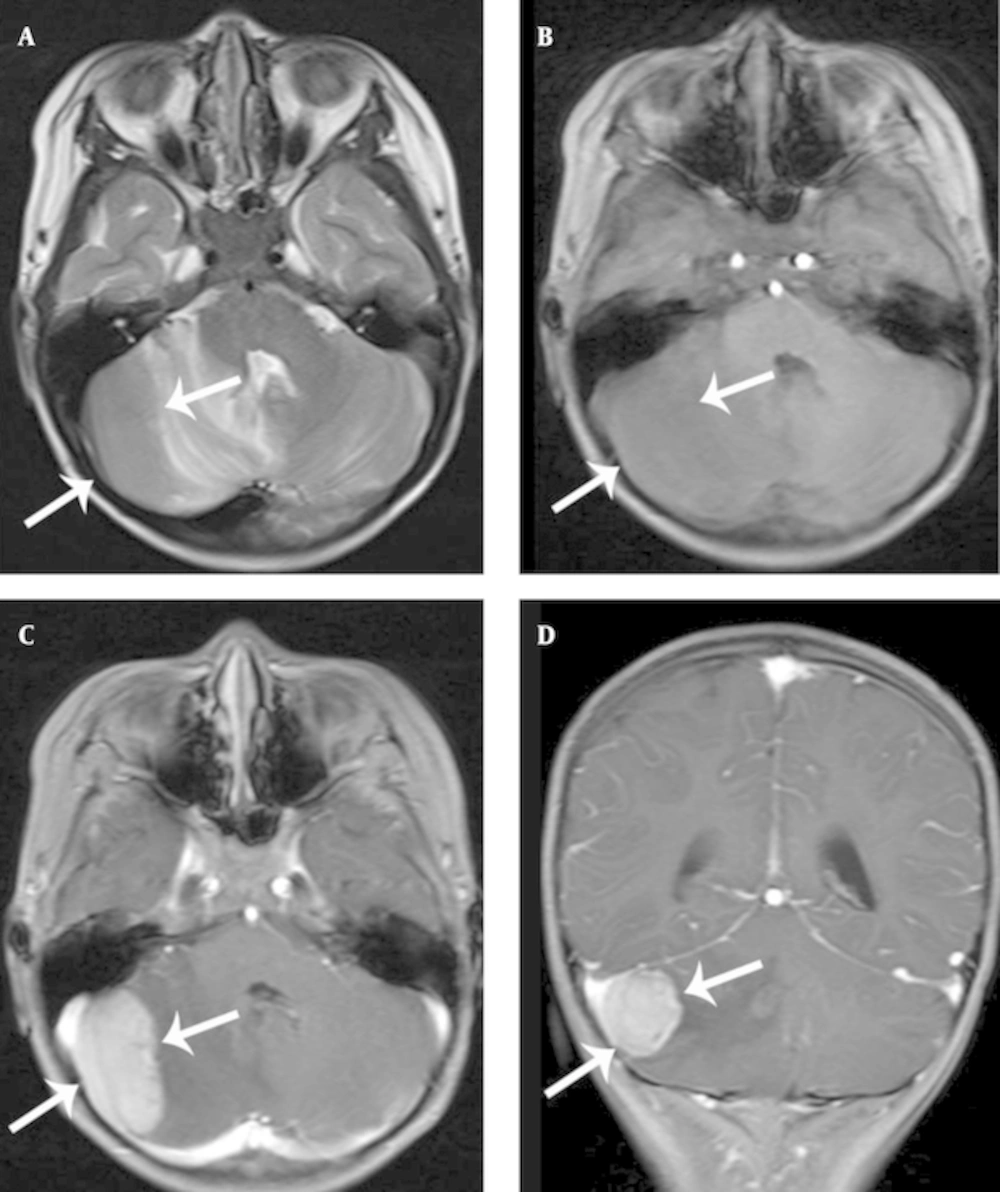
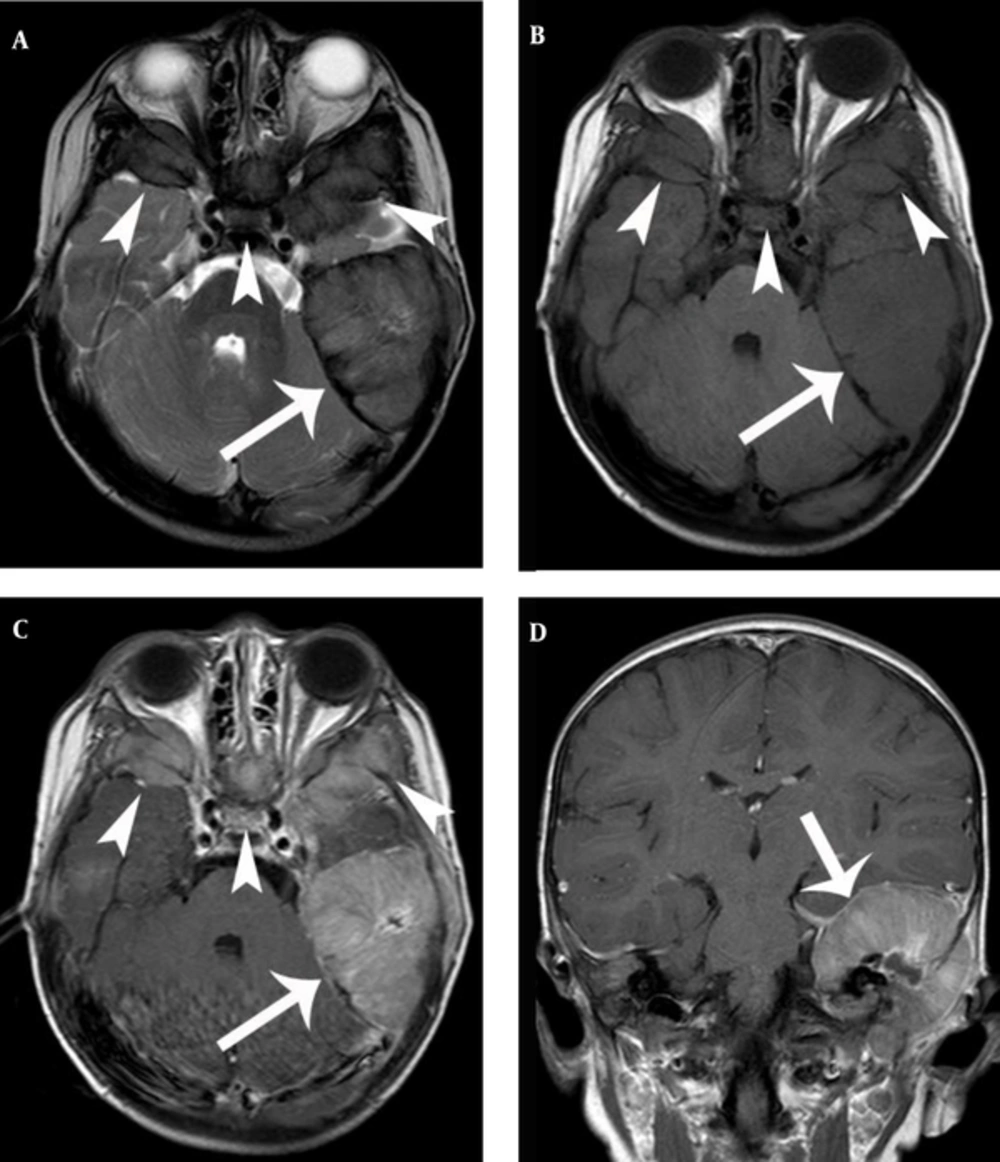
Radiologic findings were nonspecific, and a variety of structures were involved. The EMR of CNS was hypointense or isointense on the T1-weighted magnetic resonance (MR) images and isointense or hyperintense on the T2-weighted MR images. The structures were enhanced homogenously after the injection of contrast media (Figures 1 and 2).
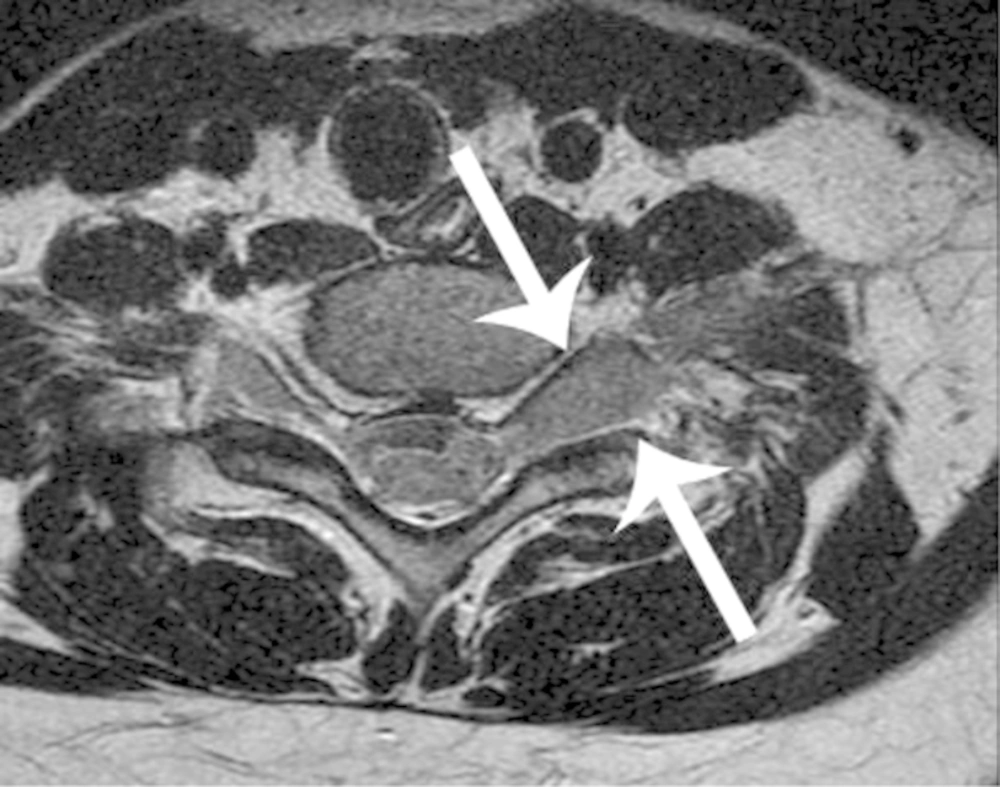
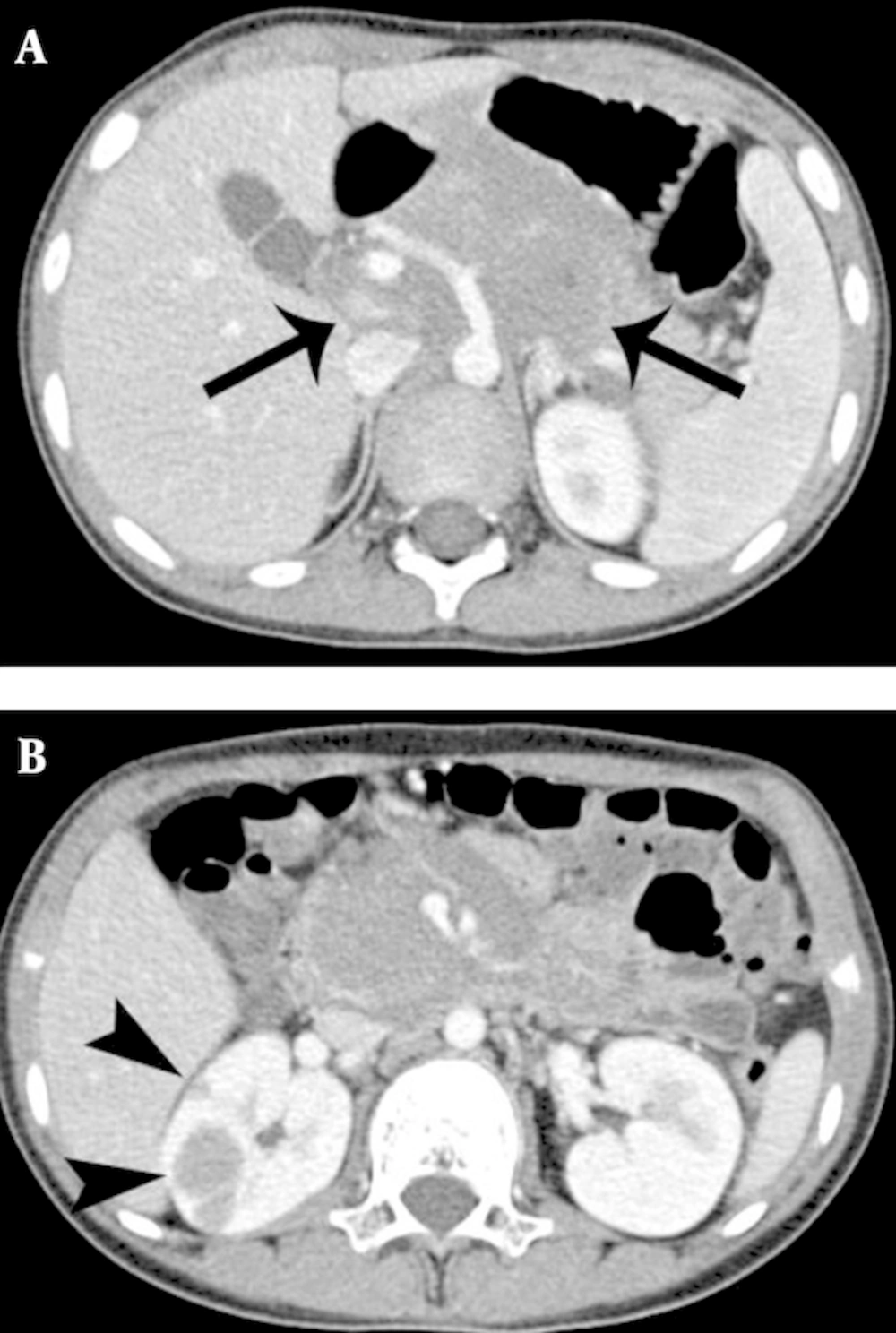
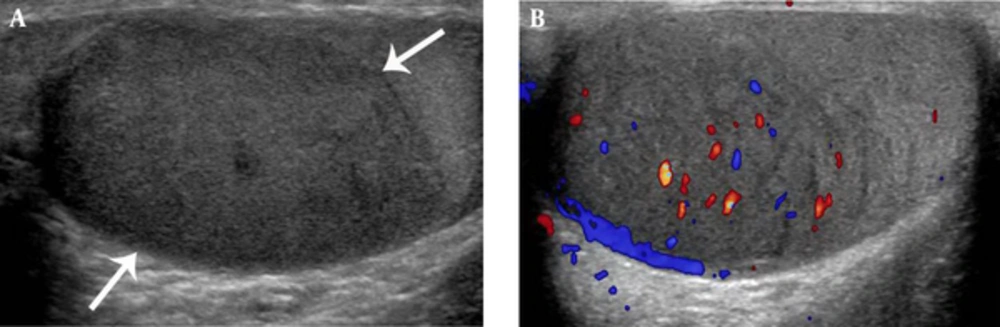
Bone and muscle lesions showed isointense and mildly hyperintense to normal muscle on T1- and T2- weighted MR images (Figure 3). Among the five cases of bone EMR involvement, four showed a permeative pattern of bone destruction with periosteal reaction on radiography. One case depicted MR images only. In the head and neck regions, EMR was presented with a bulging mass formation. Hepatic or renal involvement appeared as a well-defined solid mass, which could be indistinguishable from an abscess or lymphoma (Figure 4). Most EMR revealed discrete hypodense solid masses on the CT with a typically homogeneous enhancement (Figure 4). US helped with determining tumor extent and guiding biopsy. Echogenicity was generally homogenously hypoechoic with typical hypervascularity (Figure 5).
A, Axial T2 and B, axial T1 showing a large bulging mass on the left mastoid (arrow) and similar lesions involving the skull base and orbits (arrowheads). C, Axial T1 and D, coronal T1 with gadolinium reveal a relatively homogenous enhancement with a central non-enhancing area (arrow, arrowheads).
4.5. Clinical Course of EMR
The treatment and outcome of the patients with EMR are listed in Table 3. Seven EMR patients with concurrent BMR showed a relatively grave clinical course, and three of the seven expired. Among the 22 patients with EMR, 5 patients expired with a median of 15.1 (2.2 - 39) weeks. The CR from the EMR occurred with a median of 10.3 (2 - 20) weeks. The CR from the BMR occurred with a median of 10.3 (2 - 20) weeks.
5. Discussion
Although the prognostic significance and management of EMR in acute leukemia is controversial because of its low incidence, EMR reports have demonstrated several meaningful results (3-5, 9-12).
Factors predisposing EMR to AML include an abnormal karyotype (e.g., t(8; 21), inv(16) and t(11q23)) FAB type M4, five morphologies, and strong male predominance (3, 11). Additional EMR risk factors for ALL include male sex, age < 1 year or 10 years at diagnosis, WBC count of > 50,000/ L, T-cell lineage, and cytogenic abnormalities such as t(9; 22) (4, 5, 12). In our study, patients with EMR had a significantly higher incidence of t(8; 21) cytogenetics. The most frequent FAB morphology was M2 in AML and L1 in ALL patients. A strong male predominance (68.2%) was also found.
Patients with or without prior EMD had different EMR rates (11). However, unlike previous studies, we found no significant correlation between initial EMD at first diagnosis and EMR. In our series, only 1 patient out of 22 s with EMR had initial EMD. However, the possibility of initial EMD cannot be completely excluded because the entire body screening is not usually performed at the initial work-up for acute leukemia except for symptomatic lesions.
EMR may occur at any part of the body. The most common sites of EMR are the CNS and skin in AML and CNS and testis in ALL (4, 9, 10). The most frequent site of EMR in our patients was the CNS, but different results were found in the predominant sites of EMR patients with AML and ALL. The sites of EMR were predominantly CNS except for CSF and bone in AML (29.2% and 16.7%, respectively) and CSF and kidney in ALL (28.6% and 42.8%, respectively).
EMR may herald BMR (within 1 to 12 months), and thus a prompt EMR diagnosis is necessary (5, 13). Our series revealed a similar finding and the mean interval until BMR was 3.4 (1 - 7.2) months.
The prognosis was better known among patients with isolated EMR than among patients with concurrent BMR or later (14, 15). In our series, EMR patients had concurrent BMR in 31.8% and exhibited a relatively grave clinical course.
A low incidence of EMR may cause misdiagnosis and delay in therapy. In addition, the radiologic features of EMR are usually similar to those of lymphoma, infection, and secondary neoplasm (1, 16, 17). However, EMR should be considered whenever a mass is newly detected in a patient with a history of acute leukemia.
Our study has several limitations. First, this work was a retrospective study and was based on a small number of patients. Second, we did not have pathologic confirmation of EMR in every case.
5.1. Conclusion
In conclusion, EMR appears to have a predilection for AML in CNS except for CSF and bone and ALL in CSF and kidney. No definite relationship was found between the initial EMD and EMR. Patients with EMR had a significantly high incidence of t(8: 21) cytogenetics and FAB M2 and L1 morphologies. Knowledge of the potential sites of EMR and their risk factors may be helpful in the early diagnosis of relapse and planning for therapy. Additional studies with greater samples may be needed to define the risk factors and prognostic indicators.