1. Background
Maintenance of normocarbia may reduce the incidence of mortality and morbidity such as intraventricular hemorrhage, periventricular leukomalacia and bronchopulmonary dysplasia in newborns especially premature infants (1, 2). The measurement of blood gas carbon dioxide (CO2) level is critical tool in the assessment and principal aspect of monitoring the respiratory status specially for neonates, in particular those receiving mechanical ventilation.
Hence acid-base information obtained from blood gas samples taken from an indwelling arterial catheter appears to be the gold standard but it is difficult to obtain a sample and has important complications. Capillary and venous blood gas samplings are easier to obtain and a less invasive way of evaluating acid-base status. Both avoid the risks of arterial punctures. Several studies have shown good correlation between capillary blood, venous blood, and arterial blood gas values (3, 4). Therefore partial venous carbon dioxide tension (PvCO2) is an alternative to assess the blood carbon dioxide status in sick newborn. Obtaining venous blood sample is invasive and painful method with some complications (infections, significant blood loss). Also it provides only a single measurement of CO2 tension which is often a rapidly changing parameter (5).
The partial pressure of transcutaneous CO2 (PtCO2) is considered as an accurate estimate of both arterial and venous CO2 tension in infants and children and estimates better than end tidal carbon dioxide (ETCO2) (5, 6).
2. Objectives
The purpose of this study was to compare the PvCO2 and PtCO2 data based on separated three PvCO2 value groups (hypocapnia, normocapnia, and hypercapnia) and to understand the usefulness and limitations of PtCO2 monitoring in neonatal care.
3. Materials and Methods
This prospective and observational study was conducted in the neonatal intensive care unit (NICU) at the Sisli Hamidiye Etfal Educational and Research Hospital, Istanbul during September to December 2012. The local ethics committee approved the study and informal parental consent was obtained for each infant. Inclusion criteria were clinical indication of blood gas sampling and transcutaneous monitoring, and absence of an umbilical or radial arterial catheter. Examination was done within the first 6 to 28 days of life. All measurements were performed on mechanically ventilated infants. Infants were not studied if they were older than 28 days, had anemia, edema, hypotension requiring vasoactive drugs, hypothermia and capillary refill time of greater than two seconds, or if transcutaneous readings could not be made for any reason.
All newborns were monitored by using transcutaneous monitor (TCM TOSCA, Radiometer Medical ApS, Denmark). Once the site was cleaned with soap, 1-2 drops of contact gel was placed inside the ring and sensor fixation ring was placed on upper chest (parasternal anterior chest wall which is a highly vascularized area). The electrode was always placed on the anterior thorax, and its surface temperature was maintained at 44°C, according to manufacturer’s instructions and previous literature. Sensor calibration was automatically done after every monitoring and the membrane was changed every 14 days according to manufacturer's instructions because the electrolytes between the sensor and the membrane became depleted.
Venous blood gas sampling and monitoring of the PtCO2 level were done simultaneously. Within 5 min the venous blood gas determinations were performed using an automatic blood gas analyzer (Roche Omni C blood gas analyzer, Roche Diagnostic, Diamond diagnostics, USA). At the end of the monitoring, the transcutaneous sensors were removed and the underlying skin examined.
After collection of data, PvCO2 values were divided into three groups: hypocapnia (Group 1: < 4.68 kPa, normocapnia (Group 2: 4.68–7.33 kPa), hypercapnia (Group 3: >7.33 kPa) and then PvCO2 and PtCO2 data within each group were compared separately. The differences between PvCO2 and PtCO2 were analyzed using a Student’s paired t test. Agreement was illustrated by Bland-Altman plots with 95% limits of agreement. P-value < 0.05 was accepted to be statistically significant. A bias of more than ± 0.7 kPa was considered unacceptable (7). Data were analyzed and visualized using MedCalc program.
4. Results
Among the patients included in the study, 9 were term and 26 preterm newborns. The median gestational age was 32 (25-41) weeks, median birth weight was 1700 (780-3400) g and median age at measurement was 13 (6-25) days. Primary diagnoses of infants were prematurity with RDS (n = 19), respiratory failure (n = 11), persistent pulmonary hypertension of the newborn (n = 2), birth asphyxia (n = 2) and pneumothorax (n = 1). A total of 168 measurements of each PvCO2 and PtCO2 data were compared in three separated groups (13 readings in hypocapnia group, 118 in normocapnia group, and 37 in hypercapnia group). No infants were excluded due to instability since this was part of the study.
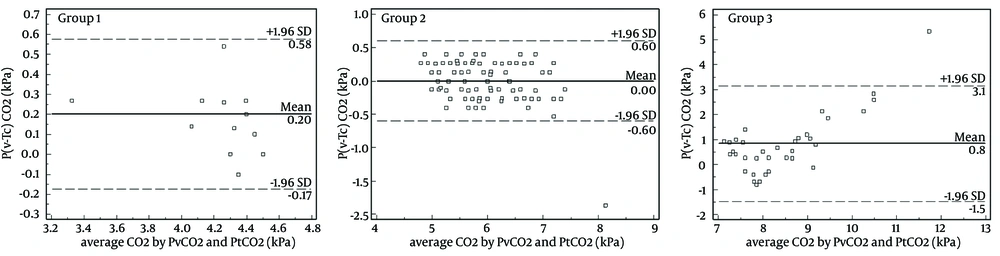
A comparison of PvCO2 and PtCO2 levels in kPa according to study groups and gestational weeks (whether term or preterm) of neonates are shown in Table 1. Although a statistical difference was detected, there was clinically a good relation between PvCO2 and PtCO2 in preterm, term, Group 1, Group 2 and all infants. On the other hand, only 37.8% of PtCO2 recordings were within 0.7 kPa of the paired PvCO2 in hypercapnia group. The difference between PvCO2 and PtCO2 (P(v-Tc)CO2) against average CO2 in the three Groups are illustrated in Figure 1. PtCO2 was related to PvCO2 with laboratory acceptable results between the two measurements in hypocapnia and normocapnia groups. All PtCO2 values were statistically significant and lower than PvCO2 data in hypercapnia group (Table 1Figure 1). The mean pH values were 7.37 ± 0.07, 7.32 ± 0.04 and 7.25 ± 0.05 in Groups 1, 2 and 3, respectively. The mean warm-up period was 9.4 ± 0.3 minutes for all patients. No serious skin lesions or any other adverse events were detected except transient mild erythema after 4 (2.3%) measurements.
| PvCO2, kPa | PtCO2, kPa | Mean difference, kPa | 95 % CI | P Value | |
|---|---|---|---|---|---|
| 6.60 ± 1.70 | 6.39 ± 1.39 | 0.21 ± 0.75 | 0.08-0.33 | 0.001 | |
| 5.83 ± 0.99 | 5.71 ± 0.87 | 0.11 ± 0.28 | 0.01-0.22 | 0.03 | |
| 4.33 ± 0.29 | 4.23 ±0.33 | 0.20 ± 0.19 | 0.09-0.12 | 0.03 | |
| 5.94 ± 0.64 | 5.93 ± 0.72 | 0.002 ± 0.30 | -0.06-0.05 | 0.938 | |
| 8.86 ± 1.56 | 8.05 ± 0.72 | 0.81 ± 1.19 | 0.41-1.20 | < 0.001 | |
| 6.46 ± 1.62 | 6.27 ± 1.26 | 0.19 ± 0.69 | 0.08-0.29 | 0.001 |
5. Discussion
Although PaCO2 remains the gold standard and PvCO2 is preferred alternative method, PtCO2 monitoring is a non-invasive technology and very valuable adjunct for respiratory management and also allows continuous monitoring (5). It is suggested that new generation transcutaneous monitors provide safe and useful carbon dioxide monitoring in newborns (8, 9). In this study close correlation was demonstrated between PvCO2 and PtCO2 values in hypocapnic and normocapnic PvCO2 level whereas for hypercapnic PvCO2 level was not.
As far as we know, this is the first study to demonstrate the relationship between PvCO2 and PtCO2. Several studies have shown a good agreement between PtCO2 and PaCO2 in newborn (10-14), although their accuracy diminished when the CO2 tension increased especially when the increase was greater than 56 mmHg (15, 16). According to our results which are similar to those reports, we cannot assume that the CO2 variations could reliably reflect PvCO2 variations in hyperkapnic newborns. Acidosis negatively affects the ability to correlate transcutaneous and venous CO2 values (5, 13, 17). In hypercapnia group mean pH value was lower than that in the other groups. So, we speculated that the capillary blood flow and gas diffusion of the skin may be even impaired when the pH decreases. This condition impairs the transcutaneous measurements and may alter the PtCO2 correlation with PvCO2.
PtCO2 measurement is based on the observation that CO2 has a high solubility and diffusion through the skin; local heat dilates blood vessel and enhances skin permeability (18). It is stated that PtCO2 measurements provide accurate results in newborns because of their thin epidermis. The epidermal layer of preterm infants is advantageous in the accurate measurement of PtCO2, but on the other hand disadvantages may cause heat induced skin damage (erythema, blisters, burns, skin tears) from the electrodes (19, 20). To achieve accurate measurements, the recommended skin prob temperature is 44°C (9). So transcutaneous CO2 measurements were carried out at 44°C electrode temperature. According to recommendation for changing sites every 2 hours to avoid thermal injury (9), we monitored the patients no longer than 2 hours and no serious adverse effects were identified except for mild transient erythema after only 2.3% of measurements.
Transcutaneous monitoring systems have some other limitations such as difficulty in keeping them calibrated, preventing air trapping and taking up longer time to sufficiently warming the skin. The need for frequent changes in sensor sites was considered breach of minimal handling approach (21). The response time decreases with elevated electrode temperature (22). In present study we chose high electrode temperature, therefore the calibration problems did not occur in our application. The average time required to heat the skin was found to be 10 minutes. It is a long time, for this reason transcutaneous measurement of carbon dioxide is not useful during the early resuscitation in the delivery room (23). Transcutaneous measurements can be difficult to use in emergency situations and not appropriate to assess the use of instant carbon dioxide level (it requires time to calibrate and warm the skin), but is suitable for follow-up and an important method for monitoring CO2 in neonates. We had no concern about the minimal handling approach, as the electrode location was not frequently altered. According to our results transcutaneous CO2 monitoring would not create a serious complication in NICU.
Our study had some limitations; it was a single center study and acceptable limits of agreement of 0.7 kPa was chosen based on previous studies (7). The measurement of PaCO2 which is considered the gold standard method was not used in our setting because of its practical difficulty. Instead of it we used PvCO2 which is commonly used paramater in clinical practice.
The present study suggests that the relationship between PvCO2 and PtCO2 is deteriorated with hypercapnic level of PvCO2. When the PvCO2 levels increase, the difference of PvCO2 and PtCO2 values also increase. Transcutaneous PCO2 measurements have generally good agreement with PvCO2 in hypocapnic and normocapnic intubated infants but there are some limitations especially for high levels of CO2 tension. We recommend that transcutaneous readings to be confirmed with blood gas values in order to verify the hypercapnic transcutaneous values and persistent or unexpected changes in PtCO2.