1. Introduction
Hemangiomas are the most common benign tumors of the head and neck in infants, comprising about 1.6% of all congenital laryngeal anomalies (1). Although present at birth, hemangiomas of the airway usually become symptomatic at 1 - 6 months of age, when their rapid growth causes increasing obstruction of the airway. The natural history of an airway hemangioma is characterized by progressive airway obstruction during the proliferative phase, followed by resolution of the symptoms during the involutive phase (2).
Currently, infantile hemangiomas of the airway are diagnosed at bronchoscopy as part of the investigation of stridor or other respiratory symptoms (3). Direct laryngoscopy usually reveals a soft, mucosa-covered, compressible, purple, blue, or pinkish lesion in the subglottic area (1).
Here, we present a submucosal subglottic hemangioma missed at bronchoscopy to show that three-dimensional computed tomography (3D-CT)/bronchoscopy can be effective in the diagnosis and follow-up of subglottic airway disease.
2. Case Presentation
A 2-month-old girl was transferred to our hospital with a history of bronchiolitis followed by sustained stridor. The infant had developed respiratory distress 20 days after birth and had been hospitalized for treatment on three occasions. Although laryngoscopy, a cardiac echogram, and brain magnetic resonance imaging were performed to evaluate her dyspnea and stridor, there was no obvious finding in any of these examinations.
The infant was born after 40 weeks gestation with a birth weight of 3.48 kg. She was born with a cutaneous hemangioma involving the right cheek, perioral, and mandibular areas. However, there was no history of respiratory distress or stridor at the time of birth. She had fed well, without difficulties.
On physical examination, a coarse respiratory sound with inspiratory stridor and subcostal retractions were noted at a respiratory rate of 37/minute. The oxygen saturation on room air by pulse oximetry was 100%. There was no abnormal finding on the plain chest radiograph.
On admission, bronchoscopy was performed under general anesthesia (IV ketamine, 2 mg/kg of body weight) by an experienced pediatric pulmonologist using a pediatric flexible bronchoscope with an outer diameter of 2.8 mm (Olympus BF 3C20). There was marked resistance as the bronchoscope entered the subglottic area. Bronchoscopy revealed a swollen vocal cord and narrowed subglottic space with superficial erosions, but no definite causative lesion.
Three days later, 3D-CT/bronchoscopy (SOMATOM Sensation 64; Siemens Medical Solutions, Germany) was performed with the patient under sedation (oral chloral hydrate, 0.5 mL/kg). We administered 2 mL/kg of a contrast agent (sodium meglumine ioxithalamate, Telebrix®; Guerbet, Aulnay-sous-Bois, France) intravenously at a rate of 0.5 - 1 mL/second via a mechanical injector (Injektron CT2; Medtron, Saarbrücken, Germany). The protocol included a scout film of the chest, a single-phase enhanced axial scan of the chest, and 3D volume-rendered (VR) imaging of the airways. Other multidetector (MD) CT parameters included a slice thickness of 3 mm, collimation of 2 mm, pitch of 1.2, gantry rotation time of 0.5 s, fixed tube voltage of 80 kVp, and automatic tube current modulation (CARE Dose 4D; Siemens) with a reference tube level of 100 mAs. The effective radiation dose was 0.65 mSv. A high-resolution algorithm was used to reconstruct external 3D volume rendering of the airways. About 20 horizontally rotated images were captured and sent to our PACS system to be reviewed.
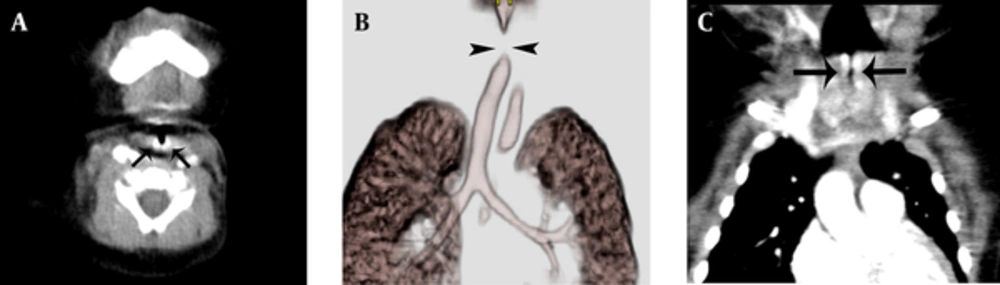
The axial CT image revealed well-enhancing wall thickening of the subglottic airway (lateral and posterior walls). The coronal reconstituted image was used to evaluate the length of the hemangioma. There was resultant airway narrowing. The 3D VR image suggested segmental non-visualization of the subglottic airway, with near-complete or complete obstruction of the trachea (Figure 1A - C).
A, the initial axial CT image shows wall thickening and intensely enhancing posterior and lateral aspects of the subglottic airway (arrows); the initial CT showed that the airway hemangioma was 2.73 mm thick; B, the resulting airway narrowing is seen as a non-visualized segment (arrowheads) of trachea that measured 4.08 mm on the initial 3D-CT/bronchoscopy; C, The resulting airway narrowing is seen as a non-visualized segment of trachea that measured 4.08 mm on the initial 3D-CT- bronchoscopy (arrows).
The patient was started on oral prednisolone 10 mg/day, and her irritability, respiratory symptoms, and auscultation were monitored. She had an excellent response to prednisolone. On the third day of treatment, she was discharged on a reduced dose of prednisolone, 7.5 mg/day.
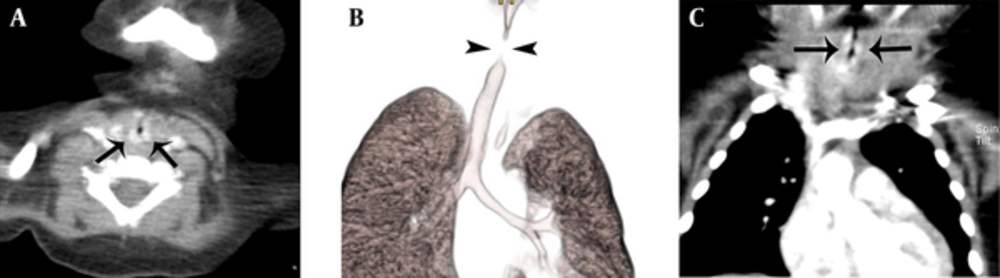
On day 17, the prednisolone was tapered to 5.0 mg/day, but shortly after the prednisolone was decreased, she developed episodes of progressive respiratory distress, which necessitated increasing the dose to 10 mg/day. On day 35, follow-up 3D-CT/bronchoscopy was performed to evaluate frequent chest wall retractions and irritability with poor weight gain. The axial and coronal images showed mild improvement of the thickness, length, and degree of enhancement of hemangioma of the subglottic airway. The 3D VR image showed a shorter length of non-visualized trachea, suggesting slight regression of the hemangioma. Subsequent bronchoscopy revealed mild improvement of the narrowing in the subglottic area and less resistance while entering the area (Figure 2A - C ).
A, follow-up CT after 40 days of treatment showed reduced thickness of the airway hemangioma to 1.94 mm with decreased enhancement (arrows); B, the length of the non-visualized segment (arrowheads) of trachea has shortened from 4.08 to 3.30 mm on the follow-up 3D-CT/bronchoscopy; C, the length of the non-visualized segment of trachea has shortened from 4.08 to 3.30 mm on the follow-up 3D-CT-bronchoscopy (arrows).
The improved respiratory symptoms combined with CT-based evidence of an involuting subglottic hemangioma confirmed the effectiveness of clinical regimen. After treating our patient for 3 months with prednisolone, nebulized budesonide, and oral propranolol, the infant showed marked regression of her respiratory distress.
3. Discussion
Subglottic hemangiomas are vascular malformations located below the level of the true vocal cords. They account for 1.5% of laryngeal abnormalities, and females are affected twice as often as males. Although 50% of children with laryngeal hemangiomas also have cutaneous hemangiomas, only 4% of those with cutaneous hemangiomas have laryngeal hemangiomas. However, there seems to be an increased risk of laryngeal hemangioma if the cutaneous hemangioma has a “beard” distribution (4), as in our case.
Subglottic hemangiomas are associated with an alarming mortality rate that can reach 50% if left untreated (5). The most sensitive form of rapid initial laryngeal assessment in a stridorous infant is nasopharyngoscopy (3). The diagnosis is made on visualizing a red or bluish compressible lesion (5).
However, one study reported that five of eight infants with subglottic hemangiomas had pink (normal) mucosa on laryngoscopy, and laryngoscopy provided no information about tumor etiology or extent (6). The correct diagnosis might be missed if laryngoscopy is performed when the child is on steroid therapy or has an endotracheal tube. An inflammatory change in the subglottic mucosa may also mimic the findings of hemangioma in patients with spasmodic croup or severe gastroesophageal reflux (6). Therefore, the diagnosis of subglottic hemangioma requires an imaging technique that produces a cross-sectional view of the lesion, such as ultrasound, CT, or MRI.
Ultrasound is not used widely in subglottic hemangiomas, although it shows an excellent diagnostic and topographical correlation with endoscopic findings. However, sonographic descriptions of many variables in the differential diagnosis of stridorous infants are not yet established. MRI is good for detecting the full extent of hemangiomas, although standard MRI coils give poor spatial resolution because of the small size of the infantile larynx (6). There are also reports that enhanced CT is an excellent diagnostic tool for evaluating subglottic airway obstruction (1). CT is an important imaging tool for evaluating children with respiratory distress, as it can differentiate vascular rings and subglottic lesions as well as other extrinsic masses involving the neck and upper mediastinum (2).
A study of 11 patients who underwent CT as part of the examination for a suspected upper airway hemangioma revealed that dynamic contrast-enhanced CT is a valuable non-invasive method for evaluating airway hemangiomas, as the CT findings were equivalent to the bronchoscopy findings (1). The study suggested that CT findings are specific enough to recommended CT as the primary diagnostic tool. This is the first reported case to establish a diagnosis of airway hemangioma that was not detected on initial bronchoscopy and followed primarily with CT.
Although ionizing radiation remains a disadvantage of CT, the use of CT for airway hemangiomas is informative (7). Our case showed that the enhanced axial and coronal scans of the chest showed the thickness of hemangioma surrounding the airway, which is crucial information for estimating the extent of an airway submucosal hemangioma. Moreover, 3D VR images can add information on the length and degree of airway narrowing.
Although laryngoscopy is a proven diagnostic tool for the compromised subglottic airway, one study reported that 63% of cases (five of eight) had normal overlying mucosa (6). There is no specific appellation for these subglottic hemangiomas, and we used the term “submucosal hemangioma” for our case. Several reports have applied “submucosal hemangioma” for cavernous hemangiomas of the gastrointestinal tract in adults (8, 9) but not for capillary hemangiomas. Because capillary hemangiomas constitute the greatest proportion of pediatric hemangiomas, our report is the first to use “submucosal hemangioma” for an airway infantile hemangioma.
We expect that 3D-CT/bronchoscopy will reduce the need for invasive laryngoscopic studies and help to diagnose submucosal hemangiomas undetected on laryngoscope. Additionally, 3D-CT/bronchoscopy will help to evaluate the extent of the lesion, degree of airway narrowing, and treatment response.