1. Background
Hereditary Tyrosinemia type I (HTI) is an autosomal recessive metabolic disorder due to deficiency of fumarylacetoacetate hydrolase enzyme. The diagnosis is based on high blood tyrosine level and abnormal urinary excretion of succinylacetone (SA) (1). According to onset of symptoms HT1 is classified in acute (< 2 months), subacute (2 to 6 months) and chronic (> 6 months) forms (2). Most common causes of death are liver failure and bleeding, hepatocellular carcinoma (HCC) and pseudo porphyric crisis with respiratory failure (3). Since 1992, the 2- (2-nitro-4trifluoro-methyl benzoyl)-1, 3-cyclohexanedione (NTBC) has offered as a new promising tool for the treatment (4, 5). This treatment prevents production of toxic and carcinogenic metabolites (6). Yet, there are few reports of the long-term prognosis (5, 7).
2. Objectives
This study reports beside its clinical and biochemical presentation, the outcome of NTBC [2- (2-nitro-4-trifloro-methylbenzoyl)-1, 3-cyclohexanedion] treatment of the disease and evaluates its biochemical markers in 16 pediatric Libyan patients.
3. Patients and Methods
A retrospective clinical study was undertaken on 16 Libyan patients with hereditary tyrosinemia type I (HTI) who were diagnosed or referred to a single center at Elkhadra hospital, one of the main referral hospitals in western Libya, which provides medical services to pediatric age groups up to 16 years old. These patients were followed during the period from October 2001 to October 2010. Our orientation was based on the presence of rickets with hepatomegaly or liver disease with high alpha fetoprotein (AFP). Two patients were detected by screening at the age of one week and 1.5 months because of older affected siblings. The diagnosis of HTI was confirmed by high tyrosine level in blood and SA urinary excretion by liquid chromatography tandem mass spectrometry (LC-MS/MS). The investigation (blood for amino acids, urine for organic acids and SA or dried blood spots for the same tests) were sent to laboratories and were done either in Tunisia, Germany, France or Lebanon. X-ray of bones, echocardiography, abdominal ultrasonography and when indicated, abdominal CT scan were monitored. Follow up visits were done iat least monthly in the first 6 months, then every 3 months thereafter. Serum tyrosine was regularly monitored. AFP and liver imaging were used as tumor markers. One patient was treated with diet protein restriction due to unavailability of NTBC on diagnosis; and 15 patients were treated with NTBC (initially 1 mg/kg/day in 2 divided doses, the dose thereafter was adjusted according to clinical response ranging 0.5 - 2 mg/kg/day) (8, 9). Prescribed diet consisted of avoiding high protein, continuing breast feeding and employing a special milk formula. The goal of the diet was to maintain the blood levels of tyrosine below 500 µmol/L (by restricted tyrosine and phenylalanine each one dose not exceed 200 mg/day). Supplementation by fat soluble vitamins D and K in liver dysfunction and phosphate in hypophosphatemia were provided.
3.1. Statistical Analysis
With SPSS program version 16 the paired samples t-test was used, P value < 0.05 was considered statistically significant.
4. Results
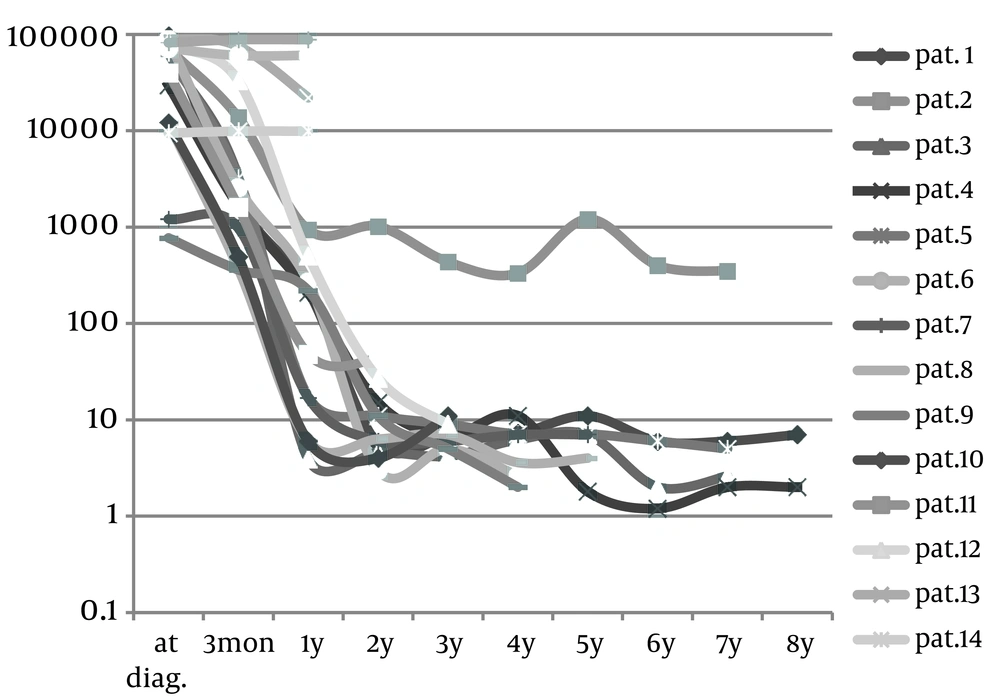
Sixteen patients were diagnosed with HTI, rate of diagnosis was 1 - 2 children/year, 81.2% (n = 13) were born to consanguineously married couples, and 2 families had 2 affected children each. The median age at onset of first symptoms was 4.5 months (range 1 week - 8 months). Median age of first visit presentation to our unit was 7 months (range: 1 week to 38 months). The median age at diagnosis was 8 months (range 1 week - 40 months). The median age at starting treatment was 9 months (range 1 week - 43 months), the median interval between diagnosis and treatment was 0.5 months (range 0 - 11 months). The main clinical presentation at first visit was hepatomegaly, jaundice, rickets with prolonged PT and hypophosphatemia which were seen in 14 (87.5%) patients. Bleeding tendency, ascites and edema with abnormal nodular liver imaging were seen in 3 (18.7%) patients with liver failure. Low corrected calcium was seen in 50% (n = 8). Low albumin was observed in 5 patients with severe rickets. Anemia and thrombocytopenia (5 patients) were transient and normalized after initiation of treatment. Other associated less common presentations were night irritability (3 patients), severe failure to thrive (3 patients), multiple fractures (1 patient) (Figure 1). No child had symptoms related to cardiovascular or central nervous system (Tables 1 and 2). At presentation, extremely high AFP (Figure 2) and high ALP were seen in all patients, the GGT was high in 87.5% (n = 14) but was normal for age in 2 patients who were diagnosed by screening, ALT was high in 68.7% (n = 11) and AST in 56.2% (n = 9) (Table 2). Overall 9-year survival rate has been 81.2% whereas it was 86.6% in NTBC treated patients.
| Variables | Values |
|---|---|
| 13 (81.2) | |
| 9 (56.2) | |
| West Libya | 10 (62.5) |
| East Libya | 6 (37.5) |
| Male | 8 (50.0) |
| Female | 8 (50.0) |
| 2 | 3 (18.7) |
| 2 - 6 | 5 (31.2) |
| 6 | 8 (50.0) |
| 15 (93.7) | |
| 3 (18.7) | |
| 4.5 (1 - 8) | |
| 7 (1 - 38) | |
| 8 (1 - 40) | |
| 9.5 (1 - 43) | |
| 9 (1 - 43) | |
| 3 (0 - 32) | |
| 0.5 (0 - 11) | |
| Hepatomegaly and jaundice | 14 (87.5) |
| Bleeding tendency (melena, epistaxis, hematamesis) | 3 (18.7) |
| Ascites, edema, nodular liver | 3 (18.7) |
| Rickets | 14 (87.5) |
Demographics of 16 Patients Diagnosed With HTI
| Variable | Median (Range) | No. (%) |
|---|---|---|
| 62014 (722 - 97425) | 16 (100) | |
| 2122.5 (772 - 4640) | 16 (100) | |
| 251 (121 - 445) | 14 (87.5) | |
| 104 (69 - 296) | 11 (68.7) | |
| 108 (49 - 316) | 9 (56.2) | |
| 5 (31.2) | ||
| 14 (87.5) | ||
| 8 (50) | ||
| 14 ( 87.5) | ||
| 3 (18.7) |
Laboratory Results of 16 Patients at Presentation a
We compared the onset of treatment and the drop of AFP levels in two groups. The first group was five patients with poor response who began treatment at median of 13 months post onset of first symptoms (range: sex to 35 months). The second group was 11 patients with good response who began treatment at median of three months post onset of first symptoms (range: one to 14 months). We observed that by the third month post treatment that level of AFP in the second group had faller by more than 50% of pre-treatment values in 90% (n.10) of patients. We observed this drop in only 20% (n.1) of the first group (P = 0.003).
The outcome of NTBC treatment after one year in 13 survived patients was as follows: PT was the first liver function marker that showed normalization (median 14 days, range 0 - 240 days), while other biochemical liver markers such as transaminases (GPT, GOT), GGT, ALP, bilirubin, serum albumin, AFP and renal function took longer to improve. In acute form all these were normal after one year of treatment. In subacute form, normalization of AFP was 50% in the first year and 100% in the second year, PT was normalized 100% and other liver markers 75%; one patient had abnormal liver imaging (hepatomegaly without nodules which sustained for longer). In chronic form, normalization of AFP was 16.6% and 83.3% in the 3rd year and later, the PT was 83.3% and other liver function markers were normalized in 66.6%; persistence of abnormal liver imaging (hepatomegaly) was seen in one patient. In none of forms increase of AFP was seen (Figure 2). The constant features were decreased rates of weight gain and poor dentition, caries or delayed eruption of deciduous teeth occurred in all patients, the first permanent teeth erupted at due time and were healthy. Neuropsychosocial development, motor function and speech were normal for age in all survived patients, but are not assessed by any standardized test system. Six patients in school age attended school without difficulties (10). Three patients who presented severe liver cirrhosis (splenomagly, esophageal varices with PT 21% to 24%) and persisting high AFP died. They were at onset of symptoms 1, 5 and 8 months, and at diagnosis 6, 6, 40 months old. The treatment was started at 7 months (diet only), 8 months and 43 months (NTBC and diet). They died at 9, 13, and 49 months, respectively.
5. Discussion
The interest for genetic disorders is relatively recent in Libya, where many sick babies or unexplained infant deaths may be attributable to inborn errors of metabolism. The preliminary investigations fail to give a diagnostic clue, the patients actually pass away without metabolic workup because lack of awareness and limited diagnostic facilities. HTI is a genetic autosomal recessive disorder; the risk is greater in mating relationships where the parents are close relatives. Libya displays some of the highest consanguinity rates in the world. According to 2008 estimate it was as high as 48.4% in a total population of 6.18 million (11). Considering that 16 patients all of whom, were referred to a single hospital, with 81.2% consanguinity rate, it seems that HTI is one of the most commonly recognized rare disorders in Libya. From other countries in North Africa, namely Tunisia, more than 70 cases were reported since 1987 with 25 NTBC treated patients since 2000 (Dr. E. Barkaoui, Tunisia International Multicenter Study 19 cases and Dr. H Azzouz, Tunisia experience in management of HTI, MEMG 2012), from Algeria 30 treated patients and Morocco 3 patients (Swedish Orphan communication). The mortality is quite high in untreated patients. Three treatment options are offered to HTI. The first option is dietary treatment that results in a poor outcome and caries a high risk of HCC, the second option is the liver transplantation which became the efficient treatment with good survey, and the third option is the NTBC which has been available since 1992, has transformed the prognosis and has obviously improved the survival rate of HTI in all forms particularly in acute liver failure. NTBC has been used since 2001 as treatment for HTI in Libya. Of patients exposed to NTBC, 60% of patients (n = 9) with more than 3 years, 40% (n = 6) with more than 6 years and 2 patients with more than 8 years of NTBC exposure. In none of the patients liver transplant was carried out. Three patients died, the cause of death was either late diagnosis or delayed starting of treatment but HCC was not ruled out (12, 13). Liver transplantation is under developing in Libya. Our next step is looking for gene mutations.
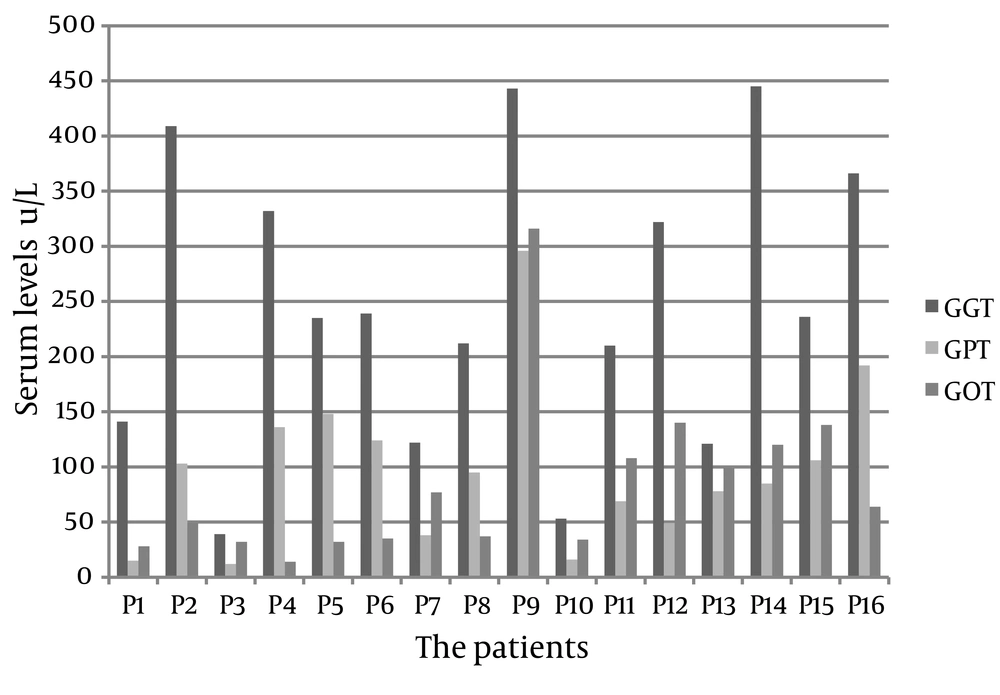
Liver transaminases are helpful in recognizing the liver disease. We found that elevated GGT was a better marker (Figure 3) with respect to following: 1) At presentation it was high and increased earlier than other enzymes in 87.5% of patients. 2) Remained longer high in the first 6 months after starting treatment except in 2 asymptomatic screened babies. 3) It was highly associated with high ALP in all patients. 4) It was the first enzyme that raised alarm when treatment in patients who previously had responded well was inadequate.
All patients showed remarkable improvement of the signs of rickets after one year of treatment. The renal tubulopathy associated with HTI treated with NTBC is usually reversible (14). No patient presented with neurological manifestation but we faced porphyric like crisis in one very well responding, early treated asymptomatic screened patient after 6 years of NTBC treatment there occurred a sudden acute shortage in NTBC, his δ-aminolevulinic acid was high, more than 20 times above the upper limit of normal, the recovery was achieved with reintroducing NTBC and supportive treatment. The side effects of NTBC, were seen in most of our patients leucopenia and thrombocytopenia, were transient but we never observed toxic signs of the eyes such as keratitis, photophobia and corneal opacities (15), nor did we observe skin exfoliative dermatitis a sign of high blood tyrosine associated with uncontrolled diet restriction.
Conclusions of the present study are as follows: 1. The study illustrates achievement of good results in Libya. 2. NTBC is well tolerated and has low adverse effects with good longterm outcome. 3. High risk groups with persisting high AFP, should be followedup for HCC. 4. GGT is a cholestatic parameter, and is the most sensitive liver enzymes marker. 5. ALP is constantly high even in asymptomatic patients and could be an early marker. 6. Fast dropping of AFP in the third month is a marker of good prognosis. 7. Introducing of neonatal metabolic screening, possibility of liver transplantation, promotion of local metabolic laboratories and a molecular testing is needed.