1. Background
Prenatal hydronephrosis (PNH) is the most common cause of kidney and urinary tract abnormality in perinatal period by a prevalence of 1% to 5% of pregnancies (1-4). Diagnosis of PNH is based on renal ultrasonography (US) before and/or after birth. In fetal US, PNH is defined as anteroposterior renal pelvis diameter (APD) ≥ 4 mm at gestational age less than 33 weeks and APD ≥ 7 mm at gestational age ≥ 33 weeks. After birth the presence of persistent urinary tract dilation seems to be the most important landmark and APD > 10 mm is abnormal (5-7).
PNH may occur by many conditions that affect kidney and urinary collecting system. Its main underlying etiologies include: transient or physiologic hydronephrosis, ureteropelvic junction obstruction (UPJO), vesicoureteral reflux (VUR), dilated ureter, multicystic dysplastic kidney, duplicated ureter. The most common cause of prenatal hydronephrosis is transient; a physiologic condition accounting for approximately 60% of cases (2, 7-9).
The intensity and severity of PNH is classified to normal (APD ≤ 9.9 mm), mild/moderate (10 mm ≤ APD ≤ 14.9 mm) and severe (APD ≥ 15 mm) according to APD of renal pelvis in US, based on society for fetal urology guidelines (2, 10-12). This grading system enables us to detect patients with serious pathology and complications or need for surgery, on the other hand, it shows the mild cases who do not need any surgical intervention and might have favorable prognosis (13-17). Outcome of PNH also relates to underlying etiology; for example, the best outcome have those with transient UPJO and dilated ureter.
Most involved infants are asymptomatic, that shows the importance of prenatal diagnosis by at least one prenatal ultrasonography. Therefore many patients recover without any surgical intervention. When symptoms arise, the finding does not improve, it may be incurable or difficult to treat (8). Type of intervention and final prognosis can be predicted based on the diameter of renal pelvis (9, 18).
2. Objectives
We conducted a retrospective study to determine the correlation between the anteroposterior diameter of renal pelvis in postnatal ultrasonography and the probability of surgical intervention.
3. Patients and Methods
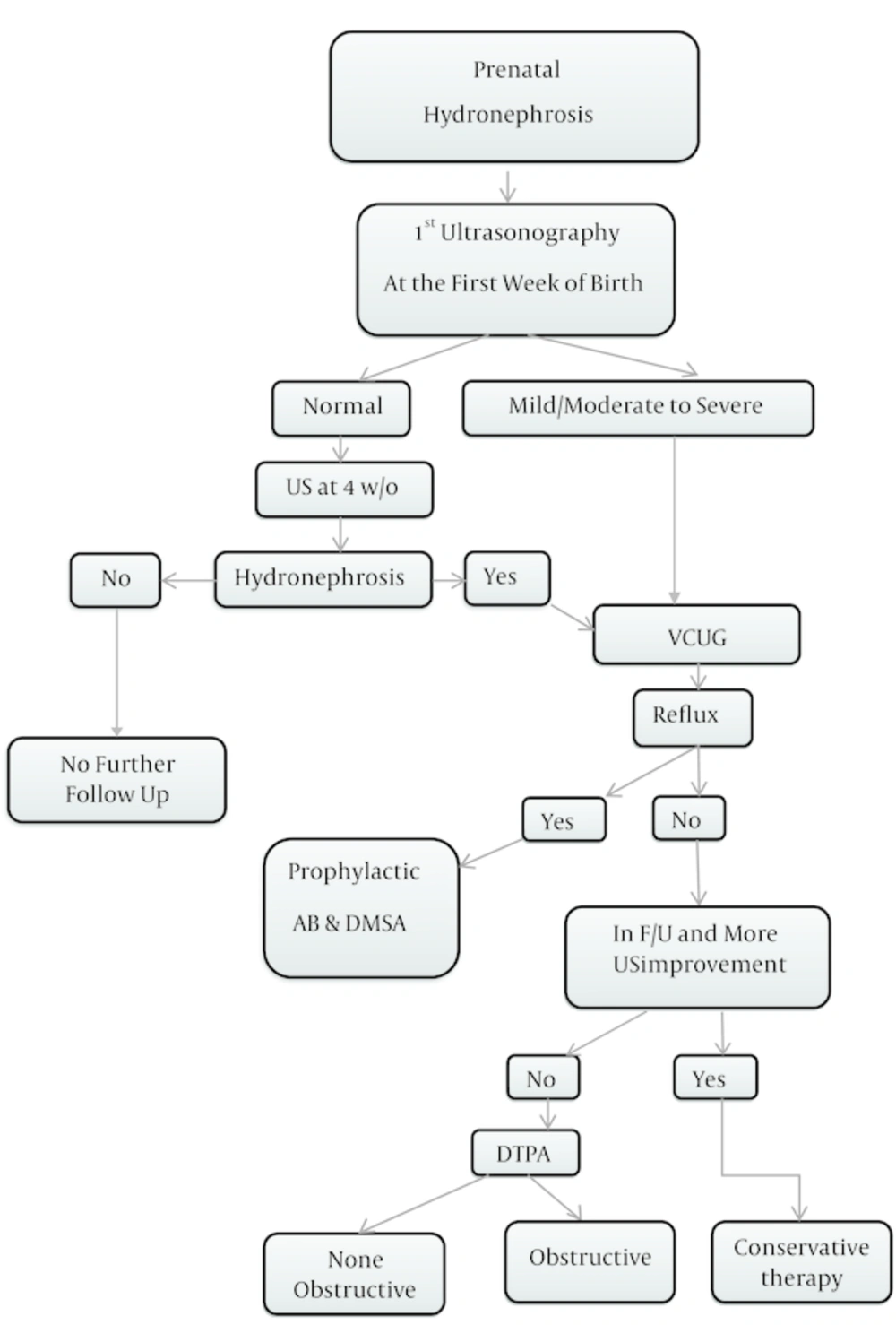
In this retrospective cohort study, infants with diagnosis PNH referred to nephrology clinic during 2011 and 2013 in Zahedan, Iran, were enrolled. PNH was defined as renal pelvis APD in ultrasonography ≥ 4 mm at gestational age (GA) > 33 weeks, and APD ≥ 7 mm at GA ≥ 33 weeks and after birth up to 2 months of age (2, 10, 19, 20). All patients with diagnosis PNH underwent at least one postnatal renal US in the first week of life by the same expert radiologist to collect comparable data without bias (21). In infants with normal 1st US, the 2nd ultrasonography was performed at 4 weeks after birth (22). Based on 1st postnatal ultrasonography our patients were classified into 3 groups; normal (APD ≤ 9.9 mm), mild/moderate (10 mm ≤ APD ≤ 14.9 mm) and severe (APD ≥ 15 mm) hydronephrosis. Most patients with mild PNH (APD ≤ 9.9 mm) had neither serious underlying pathology nor decreased renal function (15, 23), but it was necessary to re-examin them when 4 weeks old by another US. If there was no hydronephrosis, no further follow up was needed, but if hydronephrosis was presented VCUG was recommended. On the other hand, patients of severe group (APD ≥ 15 mm) had high risk of postnatal pathology and required close follow-up (10). The second step to follow-up these patients was voiding cystourethrogram (VCUG). Patients with documented vesicoureteral reflux (VUR) received prophylactic antibiotics due to increased risk of urinary tract infection (UTI) (24) and DMSA was the next modality to evaluate the renal parenchyma. Some patients with normal VCUG, who had improved in follow up and fourth week sonography, were directly put on conservation therapy with no other diagnosis modeling.
The third step was Tc-diethylene triamine-pentaacetic acid (DTPA) after 6 - 8 weeks of life in patients with normal VCUG in whom hydronephrosis was still present in follow up sonography. This modality shows obstruction in urinary tract system by delaying in kidneys spontaneous drainage. PNH with normal DTPA is considered as dilated ureter. Differential renal function 45% to 55% is normal and below 35% is significantly impaired (25-27). Other clues for obstruction is unilateral renal function ≥ 55% and prolonged drainage of 50% of radionuclide > 20 minutes (28-30). Partial obstruction was known as drainage only after IV furosemide.
All patients with mild/moderate to severe PNH received conservative management and surgery was preserved for (7, 10):
- Those with no response to conservative therapy or with posterior urethral valves (PUV) immediately.
- Any type of obstruction with renal function ≤ 35%.
- Worsening in renal function or dilation in patients with bilateral hydronephrosis or hydronephrosis in single kidney.
Other indications for surgery include pain, palpable renal mass and recurrent pyelonephritis (31).
For normal collecting system in the first control US and patients with mild hydronephrosis, second US was performed at 4 weeks of life and if no hydronephrosis existed, no further follow up was needed. If hydronephrosis was present, the next step was VCUG.
Patients were followed every 3 months during first year and every 6 months in the second year.
This study was confirmed by ethic’s committee of Zahedan University of Medical Sciences (ethic No. 91-714) and in all stages of this study, we were loyal to Helsinki declaration principles. Written consent was obtained from all of participants/parents and they were free to exit the study by their will.
Data were extracted from information forms and described in Tables 1 - 5. All data was analyzed by SPSS software (version 22). Methods have been identified in Figure 1.
4. Results
Patients presented to this study were 200 with mean age of 23.85 (range 1 to 60) days at arrival, and 78% were boys (boys to girls ratio 3.5:1). In the first postnatal US, 93% had hydronephrosis equal to 223 renal units (149 unilateral and 37 bilateral) with a mean APD of renal pelvis of 10.47 mm and standard deviation of 6.81 mm (range 3 to 44 mm).
In the first postnatal ultrasonography patients were classified into normal (65%), mild/moderate (18%) and severely (17%) affected groups.
The second ultrasonography was performed at 1 month after birth for 130 infants in whom first ultrasonography after birth was normal. The result of which was as follows: 33 infants were still normal, 99 infants showed hydronephrosis to some extent, they were conducted to the next step, namely VCUG.
VCUG was performed in 167 (83.5%) patients, equal to 57 (13 unilateral and 22 bilateral) refluxing renal units, and VUR was detected in 35 (20.83%) patients. VUR incidence in males was 68.57% and in females 31.43%. For this group of patients with positive VUR prophylactic antibiotics for UTI were started.
112 patients required to be evaluated by DTPA, 50 (25%) patients had 35 (17.5%) partial obstruction and 15 (7.5%) complete obstruction and 62 (46.43%) patients presented no obstruction; 15 patients had decreased renal function, 10 patients had non-functional kidney due to severe hydronephrosis or MCDK and 37 patients had normal kidney.
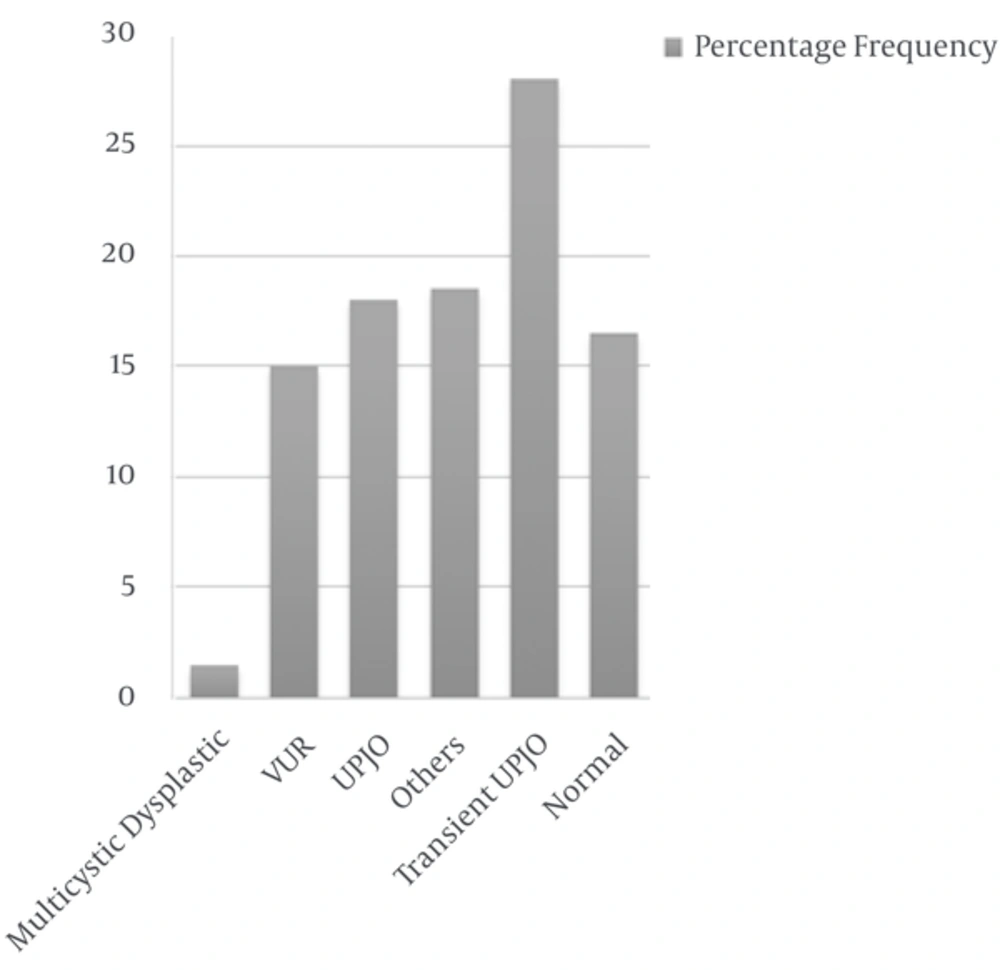
The most common (28%) cause of hydronephrosis was transient UPJO, the second cause was UPJO (18%) with unilateral to bilateral ratio 8. The third cause was VUR in 30 (15%) patients. The other causes consisted of dilated ureter (8.5%), neurogenic bladder (2.5%), duplicated ureter (2%), multicystic dysplastic kidney (1.5%), neurogenic bladder + renal agenesis, the same as ureterocele (1%) and duplicated ureter associated with UPJO or renal agenesis + UPJO (0.5%).
Duration of follow-up was variable from one month to 3 years, in average about one year. During this period, 67 (33.5%) patients had spontaneous recovery (Based on ultrasonography or VCUG) and the remaining patients received conservative therapy of whom 25 (12%) patients did not respond to medical therapy so underwent surgery; 7 (28%) patients were female and 18 patients (72%) male. The most underlying etiology resulting in surgery was UPJO (36%) and UVJO (16%). Statistical analysis (numbers and percentages) of patients is indicated in Tables 1 - 5 and Figure 2.
| Parameter | No. (%) |
|---|---|
| 200 (100) | |
| Male | 156 (78) |
| Female | 44 (22) |
| 200 | |
| Normal | 130 (65) |
| Mild/Moderate | 36 (18) |
| Severe | 34 (17) |
| 167 | |
| Normal | 132 (66) |
| Reflux | 35 (20.82) |
| Unilateral | 13 (6.5) |
| Bilateral | 22 (11) |
| 112 | |
| Non-obstructive | 62 (31) |
| Obstructive | 50 (25) |
| Partial Obstruction | 35 (17.5) |
| Complete Obstruction | 15 (7.5) |
| 200 | |
| Spontaneous Recovery | 67 (33.5) |
| Conservative Therapy | 108 (54) |
| Surgery | 25 (12.5) |
| Etiology | Total | Surgery |
|---|---|---|
| 33(16.5) | 0 | |
| 56 (28) | 0 | |
| 36 (18) | 9 (4.5) | |
| 5 (2.5) | 4 (2) | |
| 30 (15) | 5 (2.5) | |
| 3 (1.5) | 0 | |
| 37 (18.5) | 7 (3.5) |
aValues are expressed as No. (%).
bDilated ureter, neurogenic bladder, Duplex system, ureterocele, neurogenic bladder+ renal agenesis, duplication + UPJO, renal agenesis, non classified.
| Parameter | Gender | Total | |
|---|---|---|---|
| Male | Female | ||
| 20 (10) | 10 (0.5) | 30 (15) | |
| 2 (1) | 1(0.05) | 3 (1.5) | |
| 1 (0.5) | 0 | 1 (0.5) | |
| 1(0.5 ) | 0 | 1 (0.5) | |
aValues are expressed as No. (%).
| VCUG | Gender | Total | |
|---|---|---|---|
| Male | Female | ||
| 7 (3.5) | 6 (3) | 13 (6.5) | |
| 17 (8.5) | 5 (2.5) | 22 (11) | |
aValues are expressed as No. (%).
| Grad of Hydronephrosis | Gender | Total | Surgery | P Value | |
|---|---|---|---|---|---|
| Male | Female | ||||
| 102 (51.5) | 28 (14) | 130 (65) | 3 (1) | 0.00 | |
| 27 (13.5) | 9 (4.5) | 36 (18) | 10 (5) | 0.00 | |
| 27 (13.5) | 7(3.5) | 34(17) | 12 (6) | 0.00 | |
aNormal: ≤ 9.9 mm; mild/moderate: 10 - 14.9 mm; severe: ≥ 15 mm.
5. Discussion
With the widespread use of US in pregnancies the recognition of prenatal hydronephrosis has increased (2, 5, 10, 18, 32, 33). We use US to evaluate PNH. In some papers it has been emphasized that US is the best and powerful tool that is the first examination to be performed after birth (5, 34). VCUG may be safely reserved for high grade PNH (35).
In this investigation at first we used US for all patients, then VCUG and DTPA if indicated. Payabvash et al. introduced MRU to evaluate patients with congenital urogenital anomalies when other modalities are within equivocal results (36).
Boys to girls ratio 3.5:1 shows the dominance of mail in urinary tract abnormalities which is similar to the study by Bassanese et al. (Male to female ratio 7:1) (37).
Lee et al. in a study 2014 showed that out of 262 patients with PNH 18% had VUR which was the same as in our study (15%) (38).
Tombesi and Alconcher in 2012 evaluated 193 prenatal hydronephrotic newborns with APD < 15 mm and concluded that 73% of renal units resolved after one year follow up, in present study 72% of patients with APD < 15 mm recovered spontaneously or by conservative management (17).
Hydronephrosis based on first US was in 149 (74.5%) cases unilateral and 37 (18.5%) bilateral.
Anthony Herndon et al. in 1999 found that approximately 10 to 30% of cases with prenatal hydronephrosis presented with postnatal diagnosis of VUR, the majority of whom were boys with higher grades and more bilateral, completely the same as in this study (20.83% VUR, 68.57% in males) (39).
In a paper written by Ismaili et al. 33% of neonatal hydronephrotic patients had abnormal VCUG (19).
Nguyen et al. (22) in the society for fetal urology consensus statement revealed that underlying etiologies of PNH included transient hydronephrosis (41% - 88%), UPJO (10% - 30%), VUJO (5% - 10%) and VUR (10% - 20%), etc, which are similar to the results found by Lee et al. in 2006 (40) and our study.
During follow up, 25 patients of our cases needed surgery. Finding the cutoff point of APD in patients with PNH could lead to better follow-up and treatment, also it could prevent from unnecessary follow up. Several studies on PNH were done in different countries to obtain the best prediction of prognosis. These studies are designed to determine the best cutoff point of diameter of the renal pelvis leading to a surgical procedure. Similar studies have been done in the direction on fetuses and infants.Sharifian et al. in 2014 followed 178 neonates in Iran and showed that 42 (23%) patients needed surgery. In this study the best APD cutoff to predict surgery was 15 mm, the same as in our study (2).
Policiano et al. in 2014 by ultrasonography before birth showed that the APD 10 mm is strongly associated with surgery (41). In 2014, Vemulakonda et al. reported that all fetuses with any gestational age and renal pelvis diameter greater than or equal to 10 mm are known as severe PNH and need careful follow-up (18). In the article written by Safai Asl and Maleknejad the authors showed that cutoff point of APD is 10 mm in 20 weeks gestational age, 12 mm in 30 weeks gestational age, and 15 mm postnatal had most communication with the next pyeloplasty (42). Study on 178 PNHs showed that specific cutoff point 15 mm indicates the surgical pyeloplasty (2). Tombesi and Alconcher considered mild PNH and concluded only 1.5% of patients required surgery treatment (17). In the same paper, these percentages are respectively 70% and 2.4% (43). The authors, in the paper entitled “Incidence and outcomes of antenatally detected congenital hydronephrosis” reported that the best predictor, for incurable PNH and the possibility of underlying pathology (such as anatomic abnormalities) in postnatal anteroposterior diameter equal to 5 mm had a sensitivity of 83%, equal to 7 mm had a sensitivity of 88% and 10 mm had a sensitivity of 94% (20).
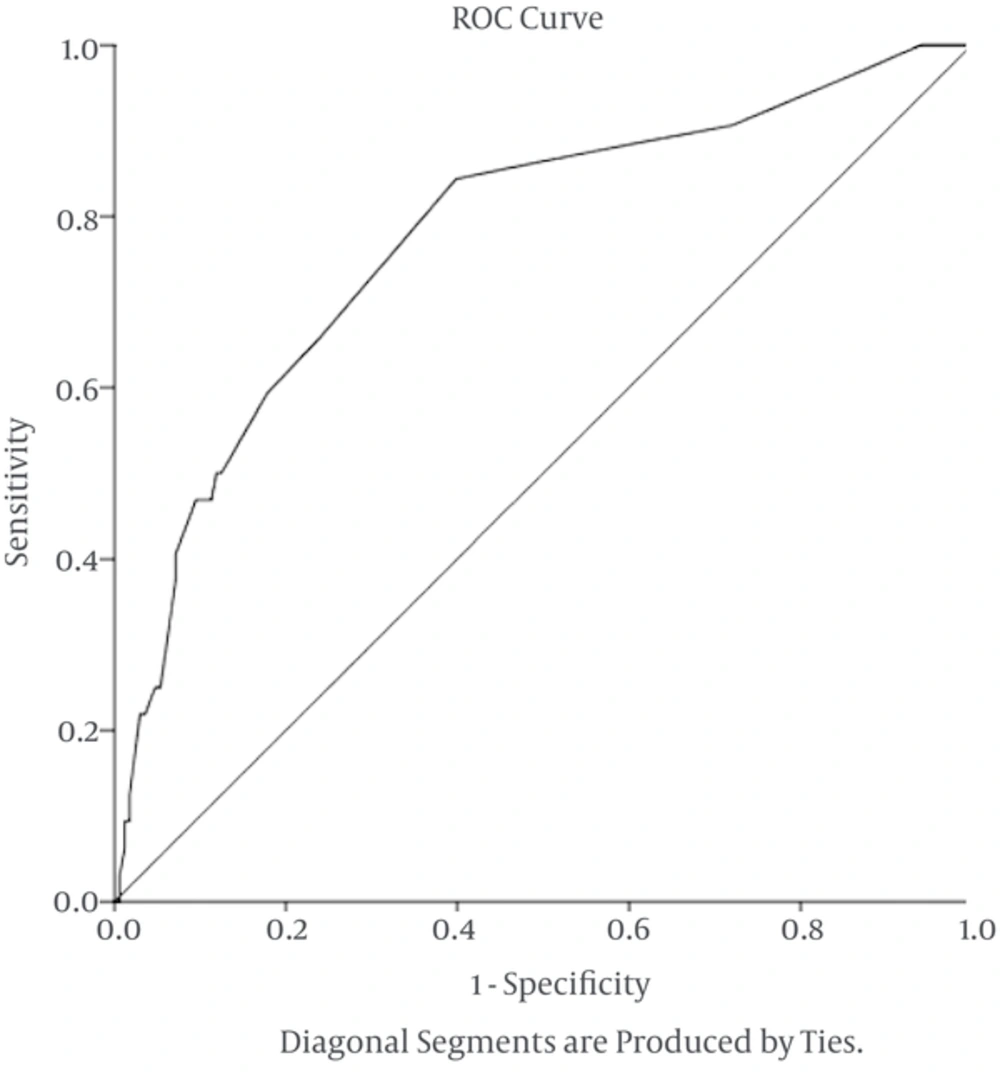
Mudrik-Zohar et al. in an article stated that APD > 14 mm is the best cutoff point for surgery with sensitivity of 77% and specificity of 69%, using the ROC curve 0.817 (44).
In the present study, 200 PNH cases were enrolled, the best APD leading to surgical treatment was15 mm with the sensitivity of 88% and specificity of 74% (Figure 3).
5.1. Conclusions
As PNH is an asymptomatic disease in early stages of life and its screening with US is very important, we showed an APD cutoff about 15 mm in the first week after birth in US is the most leading issue to a surgical outcome, thus less than that point means to have a successful conservative therapy.