1. Background
M. pneumoniae is one of the major pathogens of community-acquired pneumonia in children all over the world (1-3). M. pneumoniae easily attaches to ciliated epithelial cells of the respiratory tract and causes damage (4, 5). For diagnosis of M. pneumoniae, culture and serological diagnosis are common methods in clinical practice, but insensitivity, time-consuming or cross-reactions limit their application (6-10). Recently, PCR method was used to directly detect M. pneumoniae DNA, which has high specificity and sensitivity in clinical detection of M. Pneumoniae (11, 12). But in clinical practice, co-infections are common in children with pneumonia and direct detection of M. pneumoniae is not efficient in distinguishing it from pneumonia (8). Our previous study suggested that Th1/Th2 balance plays a significant part in anti-infectious immunity and Th1/Th2 cytokines are useful biomarkers in diagnosis and treatment of bacterial and viral infection (13-15).
2. Objectives
In this study, we wanted to confirm the value of Th1/Th2 cytokines in diagnosis and treatment of M. pneumoniae pneumonia.
3. Patients and Methods
Subjects: From May 2012 to June 2014, a total of 13161 throat swab specimens were collected. The age of the 13161 patients ranged from 3 months to 10 years. All these children had been primarily diagnosed with pneumonia (16) and had received no clinical treatment.
Detection of M. pneumoniae: M. pneumoniae PCR kit (Daan Gen Co., Ltd. Guangzhou, China) was used in DNA extraction and M. pneumoniae DNA detection (4). Amplification and data analysis were carried out with an applied biosystems 7500 real-time PCR system (Applied Biosystems, Inc., CA, USA) under this condition: 93°C for 2 minutes and 40 cycles of 93°C for 45 seconds and 55°C for 60 seconds. Specific tests were used to detect other pathogens, such as immunofluorescence to detect respiratory viruses including adenovirus, human metapneumovirus, respiratory syncytial virus, parainfluenza virus and influenza virus; blood and sputum culture for bacteria; and real-time PCR to detect human cytomegalovirus, Epstein-Barr virus, Chlamydia pneumoniae, Ureaplasma urealyticum and Chlamydia trachomatis. If any of these assays was positive, the patient was excluded from the study.
Cytokine determination: The protocol of cytokine measurement was reported in our previous study (13) as follows: The clotted blood samples were centrifuged at 1,000 g, 20°C for 20 minutes. After that, the supernatant was collected and the levels of Th1/Th2 cytokines by 320 flow cytometry detected. IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ were detected quantitatively by CBA kit-BDTM CBA Human Th1/Th2 Cytokine Kit II (BD Biosciences, San Jose, CA). After collecting the sample data on a FACScaliburTM flow cytometer (Becton Dickinson, San Jose, CA, USA), we used the BD CBA Software (BD Biosciences, San Jose, CA, USA) to display the results in tabular and graphical format. Then we established the standard curve for each reagent. 1.0 pg/mL was the lowest detection limit for these six cytokines, while the highest was 5,000 pg/mL.
Statistical analysis: We used ϰ2 or Fisher’s exact test and Mann-Whitney U test in SPSS Statistics 19.0 software. It was considered that P < 0.05 is statistically significant. We used receiver operating characteristic (ROC) curve to estimate the value of cytokines in diagnosing M. pneumoniae pneumonia by SPSS Statistics19.0 software.
4. Results
Patients’ characteristics: Between May 2012 and June 2014, 13161 throat swab samples were collected and tested for M. pneumoniae, including 1353 from outpatients and 11808 from hospitalized children. 8082 samples were from boys and 5079 from girls, yielding a male-to-female ratio of 1.59:1. 2188 were tested positive for M. pneumoniae, with a positive rate of 16.62%. Among 2188 M. pneumoniae positive samples, 1277 were from boys and 911 from girls, giving positive rates of 15.80% in boys and 17.93% in girls.
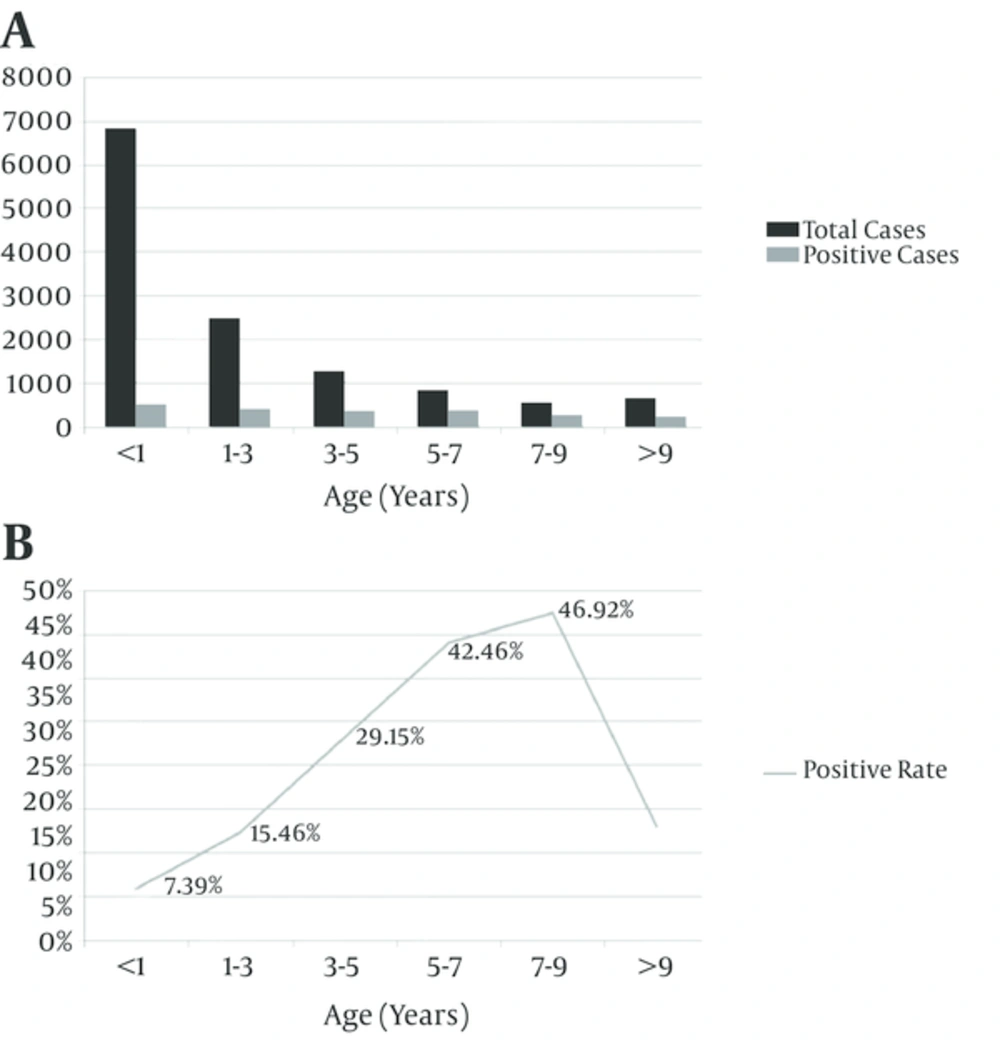
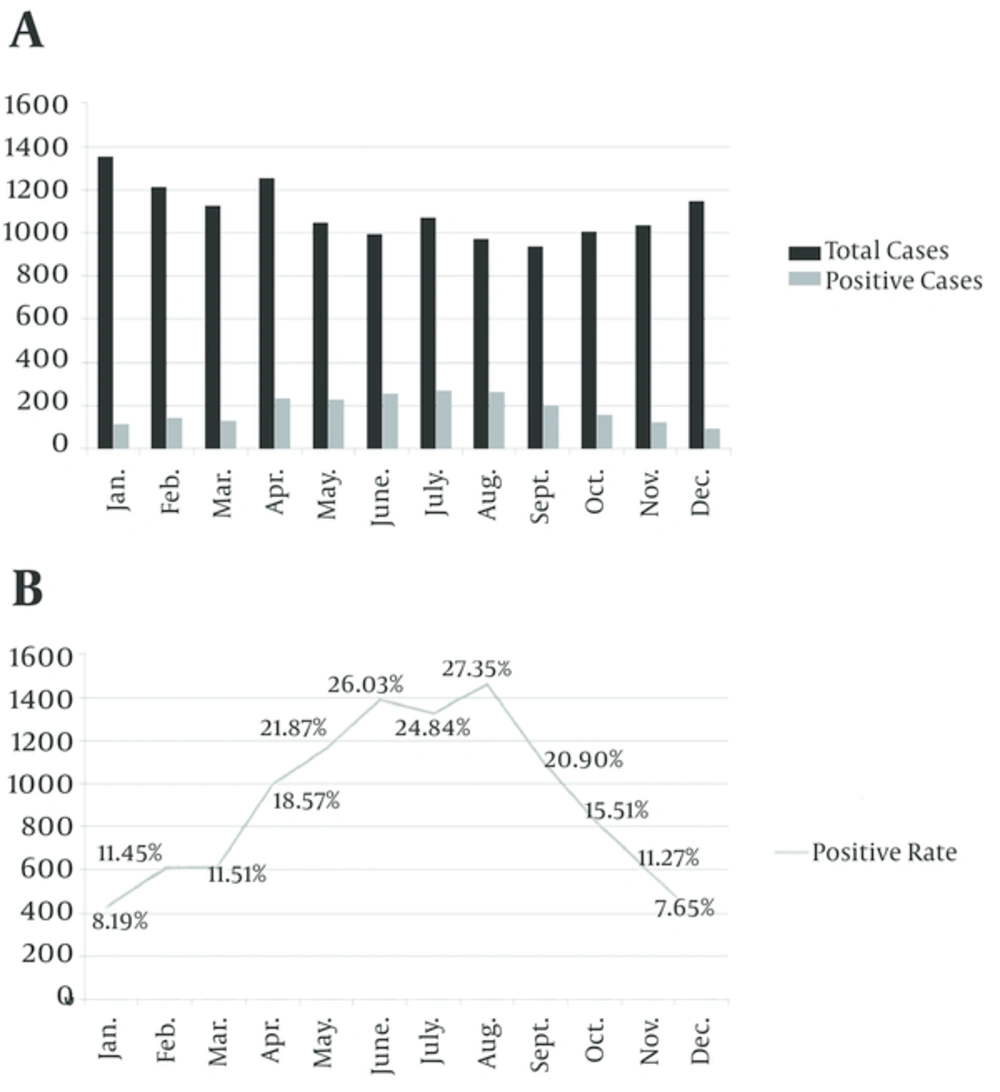
As shown in Figure 1, positive rate for M. pneumoniae infection was at the peak in children aged 5 - 9 years (42.46% - 46.92%, compared with other group, P < 0.01). It steadily declined with increasing or decreasing age. Among age groups, children younger than 1 year had the lowest (7.39%) positive rate, compared with the other group (P < 0.01). M. pneumoniae infection occurred all year round, the monthly positive rates for M. pneumoniae infection ranged from 7.65% to 27.35%, with a peak in June and August (compared with other group P < 0.01), and steadily declined in the previous and the following months (Figure 2). Co-infections were found in 1662 (75.96%) M. pneumoniae positive children, which were higher than in mono-infection children.
Inflammatory cytokine levels: To evaluate the levels of these six cytokines in healthy children and children with M. pneumoniae pneumonia. 526 patients with single M. pneumoniae infection were used as M. pneumoniae pneumonia group and 30 healthy children acted as the control group. As shown in Table 1, comparisons between M. pneumoniae infection group and normal control group revealed no difference of inflammatory cytokine (IL-6 and TNF-α) levels between the two groups (median levels, pg/mL: IL-6:14.27 vs. 4.54, P = 0.057; TNF-α: 3.56 vs. 2.21, P = 0.182). The level of IL-2 was significantly lower in serum from M. pneumoniae pneumonia patients than in serum from normal controls (median levels, pg/mL: IL-2: 3.19 vs. 5.72, P = 0.00), while the levels of IL-4, IL-10 and IFN-γ in M. pneumoniae pneumonia patients were significantly higher than in the normal controls (median levels, pg/mL: IL-4: 3.23 vs. 1.46, P = 0.00; IL-10: 5.56 vs. 2.53, P = 0.001; IFN-γ: 20.35 vs. 4.83, P = 0.001).
| Parameters | MPP (n = 526) | Control (n = 30) | P Value |
|---|---|---|---|
| 3.2 (1.0 - 7.0) | 5.7 (2.7 - 7.8) | < .001 | |
| 3.3 (1.3 - 6.3) | 1.5 (1.0 - 2.1) | < .001 | |
| 54.3 (1.0 - 1459.0) | 4.5 (1.2 - 8.5) | .057 | |
| 5.6 (1.0 - 49.9) | 2.5 (1.3 - 3.7) | .001 | |
| 3.6 (1.0 - 133.8) | 2.2 (1.3 - 3.1) | .182 | |
| 20.4 (3.0 - 158.5) | 4.8 (3.3 - 7.8) | .001 |
Abbreviations: Control, healthy children group; MPP, M. pneumoniae pneumonia group.
ROC-analysis: To confirm value of inflammatory cytokines in diagnosis of M. pneumoniae pneumonia, we used ROC-analysis to evaluate the abilities of the six cytokines in identifying the possibility of M. pneumoniae pneumonia. The AUCs were 0.922 (95% CI, 0.878 to 0.966), 0.954 (95% CI, 0.928 to 0.979), 0.819 (95% CI, 0.758 to 0.880) and 0.928 (95% CI, 0.892 - 0.963) for IL-2 (lower than 4.5pg/mL), IL-4 (greater than 2.5 pg/mL), IL-10 (greater than 3.0 pg/mL) and IFN-γ (greater than 5.5 pg/mL) respectively, with sensitivity and specificity above 80% (Table 2). These results indicated that IL-2, IL-4, IL-10 and IFN-γ could be effective biomarkers to identify M. pneumoniae pneumonia.
| Cytokines | AUC | 95% CI | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| .922 | .878 - .966 | 88.1 | 84.8 | |
| .954 | .928 - .979 | 81.9 | 100 | |
| .729 | .678 - .780 | 62.8 | 95.3 | |
| .819 | .758 - .880 | 86.3 | 82.1 | |
| .702 | .629 - .775 | 59.3 | 78.8 | |
| .928 | .892 - .963 | 80.2 | 93.9 |
5. Discussion
M. pneumoniae pneumonia is common in children, In clinical practice it is important to accurately diagnose M. pneumoniae infection (1, 2). In this article, we used real time PCR to detect M. pneumoniae in throat swab samples. Our results showed that positive rate for M. pneumoniae was highest among children aged 5 - 9 years and summer was the M. pneumoniae season in China. There are common methods to detect M. pneumoniae in clinical practice, such as PCR, culture and serological test. Interestingly, co-infections were found in 1662 (75.96%) of the M. pneumoniae positive cases. It was difficult to estimate the true association between the clinical manifestations and M. pneumoniae infection (8). So, in this study only children with M. pneumoniae infection were evaluated with Th1/Th2 cytokines level. Our previous study has shown that the useful biomarkers to diagnose bacterial infection in children were IL-2, IL-6 and IL-10 (14). In contrast to the normal controls, the levels of IL-2, IL-4, IL-10 and IFN-γ in children with M. pneumoniae pneumonia had significantly changed and had high sensitivity and specificity. Among them, IL-2 can fight against infection and plays a unique role in generating and maintaining regulatory T-cells (15). Therefore, IL-2 often decreases at the time of and after infection. After M. pneumoniae infection, changing IL-2 level was reported in previous studies with controversial results (16, 17). Our study supports findings indicating that IL-2 level decreases after M. pneumoniae infection in children. IL-10 has pleiotropy, which can limit antigen-presenting cell function and primarily inhibits antigen-presenting cells from releasing chemokines and proinflammatory cytokines (13, 14). Furthermore, it also can directly suppress T-cell proliferation, function and cytokine production to limit inflammation (14). IL-10 increases and plays an important role as anti-inflammation agent (18, 19). This is the underlying mechanism of regulating IL-2 and IL-10 during M. pneumoniae infection in children. Cytokines which are produced by Th1 cells can be blocked by IL-4 produced by Th2 cells (18). IL-4 also plays a vital role of switching to IgE which induces allergic diseases (20, 21). Additionally, increase of IFN-γ during M. pneumoniae infection was also shown in a previous report (22).
M. pneumoniae is a most common pathogen which causes pneumonia in children in Hangzhou, China. Our study suggests that the effective biomarkers which can be used to diagnose whether children with pneumonia are afflicted by M. pneumoniae are IL-2, IL-4, IL-10 and IFN-γ.