1. Background
Pediatric chronic constipation is often defined as having bowel movements less than three times per week and lasting for more than months (1). Constipation is one of the most common gastrointestinal (GI) complications, especially in the developed countries (2). The incidence of this disorder is about 15% in communities (3). Excluding neonatal period, the most common type of constipation is functional constipation (4). Chronic functional constipation (CFC) is an annoying disorder for both parents and children (5). Predisposing factors for childhood constipation may be discussed as genetic, psychological or organic (6). Common risk factors for functional constipation in children include low fiber diet, emotional stress, and positive family history in the first-degree relatives as well as painful defecation (7). Increased parental understanding and support as well as improving toileting habits and using laxatives warrant the control of this distressing condition in children (8).
Single drug therapy hardly leads to control the symptoms in afflicted children. The treatment protocol should include lifestyle change via parental education, long-term maintenance therapy and fecal mass removal (9). Polyethylene glycol (PEG) is often mentioned as the first-line treatment for children with CFC (9). PEG is a non-toxic, hydrosoluble, high-molecular polymer which is safe for long-term application in children (10).
Flixweed (Descurainia sophia L.) (D. sophia) is a known commonly-used medical herb in Traditional Persian Medicine. D. sophia is considered as a safe and mild laxative (11), and an anti-inflammatory, analgesic and anti-helmetic herbal medicament (12). D. sophia is reported not toxic at the level of up to 2500 mg/kg of body weight (13). About 15 aminoacids, 10 fatty acids, flavonoids and phenolic constituents have been isolated from D. sophia seed (11). A study showed that a combination of D. sophia and prune (Prunus domestica L.) could prevent adult Hajj pilgrims’ constipation (14). In Traditional Chinese Medicine, flixweed seeds are used to relieve cough and reduce edema. It has revealed cardiotonic effects and is also applied in some cancers. D. sophia has shown cytotoxic activity against cancer cells (15).
2. Objectives
Current study aimed to compare the efficacy of D. sophia compared to PEG in the symptomatic alleviation of pediatric constipation.
3. Patients and Methods
3.1. Trial Design
The study was conducted from January to August 2013 at Shahid Motahari Polyclinic in Shiraz. The study was approved by Ethics Committee of Tehran University of Medical Sciences; the trial enrollment started at January 2013. Before enrollment, informed consent was obtained from the parents. In this trial, patients were referred by a pediatric gastroenterologist who had done precise examination and confirmed the CFC.
3.2. Patients
Patients were selected from 221 children referred to a pediatric gastroenterologist in Shahid Motahari Polyclinic. The inclusion criteria were children between 2 and 12 years who met the Rome III criteria for functional constipation, and were free of other medications. The inclusion criteria were based on the existence of 2 or more Rome III criteria for functional constipation for at least 3 months before diagnosis and lack of the Rome III criteria for IBS (Box 1) (16). Children having organic causes of defecation disorders such as Hirschsprung’s disease, spina bifida occulta, hypothyroidism, cystic fibrosis, neurologic abnormalities, intestinal pseudo-obstruction, and diabetes mellitus were also excluded from the study (7).
Inclusion Criteria for Diagnosis of Functional Constipation in Children
3.3. Study Procedure
Through a block randomization, patients were divided into two parallel therapeutic groups (60 in each group). The sample size was determined based on similar studies (9). Totally, 120 children aged 2 - 12 years old who met the Rome III criteria were enrolled in the study. One group received an oral solution containing D. sophia seed and the other, PEG 40% without electrolyte. Due to the different tastes and appearance of both medications, the study was not blinded. However, the methodologist and the statistician who assessed and analyzed the data were blind to the protocol. At enrollment, a complete history was taken and physical examination done by a physician involved in the study. Stool frequency, fecal soiling (encopresis), stool consistency, retention posturing and abdominal pain, flatulence or rectal bleeding were recorded. Patients with fecal impaction were treated with bisacodyl suppositories at the first visit. Subsequently they were enrolled in the study.
Medications were oral administration of PEG (40% solution without electrolytes), 0.4 g/kg (17), and D. sophia, two grams for 2 - 4 years old and three grams for 4 - 12 years old patients once daily for 8 weeks. Parents were requested to refer to the on call physician in case of seven days lack of bowel movement or confronting with any other complication. During the first three weeks, parents were asked to record a daily report of defecation frequency, stool consistency, soiling, painful defecation, retention and passing blood with defecation. Additionally, weekly reports were taken from by the interviewer. At the end of the first three weeks, parents were called to describe the condition of their children. Patients were also revisited and their charts were reviewed. The efficacy of the intervention in both groups was evaluated at three weeks and eight weeks of follow-up.
3.4. Behavior Modification
According to conventional medicine and traditional medicine resources, behavior modifications and nutritional advices were the same in both groups. These points included toilet training, abstaining from junk foods and drinking adequate water, but not with the meals. The patients were asked to chew food morsels slowly (9, 18). Following termination of three weeks, patients were again visited and parents were assured to continue the behavior modifications. Medication was continued for further five weeks after the agreement.
3.5. Outcome Measures
The primary outcome was determination of proportion of patients who had responded to treatment. Response to the treatment was defined as improvement of constipation for at least three bowel movements, soft stool and convenient defecation, no soiling and bloody stool per week as well as exiting the Rome III criteria for constipation after the third week. Patients were ruled out from the study if they had no bowel movement for seven days or developed fecal impaction, at any stage.
Secondary outcomes were as follows: If the patients did not meet Rome III criteria at the end of the eighth week, the incidence and severity of gastrointestinal adverse effects including flatulence, abdominal pain and drug compliance were monitored at the end of the third and eighth week.
3.6. Statistical Analysis
Quantitative data were presented as Mean ± SD and No. (%), or MED (IQR). Statistical analyses included the determination of Means ± SD, t test, Chi-square test, Fisher exact test and Mann-Whitney U-test with a significance level of 5%.
4. Results
Among 221 referred patients, 120 cases who met the Rome III criteria for constipation were enrolled in the study. After receiving the written consents and block randomization, 11 patients withdrew the consent in the first week of treatment. The reason was doubts on the efficacy of treatment. On the other hand, 109 patients continued the recommended treatment (PEG: 53, and DS: 56). All patients were visited at the end of the third and eighth week. The participants’ age was between 2 and 12 (5.01 ± 2.38). The two groups were similar in demographic and Rome III criteria before starting treatment (Table 1).
Duration of constipation was more than six months in 105 (96.3%) and 3 - 6 months in 4 (3.7%) patients. Fifty five (98.2%) children in D. sophia and 50 (94.3%) in PEG group had a history of constipation for more than six months. Thirty eight (67.9%) patients in D. sophia and 18 (34%) in PEG group had a history of receiving multiple none controlled- medications for over six months.
| Variable | DS (n = 56) | PEG (n = 53) | P value |
|---|---|---|---|
| 4.86 ± 2.25 | 5.184 ± 2.51 | 0.480 | |
| 17.50 ± 8.23 | 19.362 ± 9.52 | 0.278 | |
| 26 (46) | 22 (41.50) | 0.373 | |
| 51.12 ± 4.46 | 47.86 ± 4.88 | 0.623 | |
| 2 (1 - 3) | 3 (2 - 3) | 0.139 | |
| 2 (1 - 3) | 2 (1 - 3) | 0.242 | |
| 2 (1 - 3) | 3 (2 - 3) | 0.162 | |
| 0 (0 - 0) | 0 (0 - 0) | 0.996 | |
| 0 (0 - 1) | 0 (0 - 1) | 0.485 |
4.1. Treatment Success
Based on Rome III criteria, both interventions demonstrated similar efficacies in relieving symptoms of constipation at the end of the third week (58.9% in the D. sophia group versus 54.7% in PEG group). However, 64.3% of patients in D. sophia group were out of criteria at the end of the eighth week as compared to those in PEG group (54.7%). No statistically significant difference was found between the efficacy of both interventions in the treatment of constipation at the end of third and eighth week of intervention (Table 2).
| Variables | DS (n = 56) | PEG (n = 53) | P Value |
|---|---|---|---|
| 1 (1.8) | 3 (5.7) | 0.288 | |
| 30 (53.6) | 30 (56.6) | 0.45 | |
| 33 (58.9) | 29 (54.7) | 0.401 | |
| 36 (64.3) | 29 (54.7) | 0.205 |
4.2. Stool Frequency
Median weekly stool frequency in the 0, 1, 2, 3 weeks of treatment was 2, 5, 5, 5 in D. sophia and 3, 4, 4, 5 in PEG group (P = 0.139, 0. 076, 0.844, 0. 294), respectively. In regard of 6 - 12 year old children at the end of the first and second week of treatment, stool frequency was significantly improved in D. sophia group, as compared to the PEG group [D.sophia group: 7 (5.75 - 7), 7 (5.75 - 7); PEG group: 3 (3 - 4), 4 (3 - 6.5), P = 0. 014; P = 0. 001]. At the end of the third week, stool frequency in the PEG group was found better than that in the D. sophia group. However, the difference was not statistically significant [D. sophia group: 4.5 (5.75 - 7); PEG group: 6 (5.75 - 7); P = 0.203] (Table 3).
| Frequency, wk | Before Intervention | After First Week | After Second Week | After Third Week |
|---|---|---|---|---|
| DS | 2 (1 - 3) | 5 (3 - 7) | 5 (3 - 7) | 5 (3 - 7) |
| PEG | 3 (2 - 3.5) | 4 (3 - 6.5) | 4 (3 - 7) | 5 (4 - 7) |
| 0.139 | 0.076 | 0.844 | 0.294 | |
| DS | 2 (1 - 3) | 2 (0 - 3) | 2 (0 - 3) | 1 (0 - 2.75) |
| PEG | 2 (1 - 3) | 2 (1 - 3) | 1 (0 - 3.5) | 2 (0 - 3) |
| 0.242 | 0.566 | 1.000 | 0.180 | |
| DS | 2 (1 - 3) | 1 (0 - 2.75) | 1 (0 - 2) | 0.5 (0 - 2) |
| PEG | 3 (2 - 3) | 2 (0 - 3) | 1 (0 - 2) | 0 (0 - 3) |
| 0.162 | 0.183 | 0.977 | 0.899 | |
| DS | 0 (0 - 1) | 0 (0 - 0) | 0 (0 - 0) | 0 (0 - 0) |
| PEG | 0 (0 - 1) | 0 (0 - 0) | 0 (0 - 0) | 0 (0 - 0) |
| 0.485 | 0.503 | 0.845 | 0.180 |
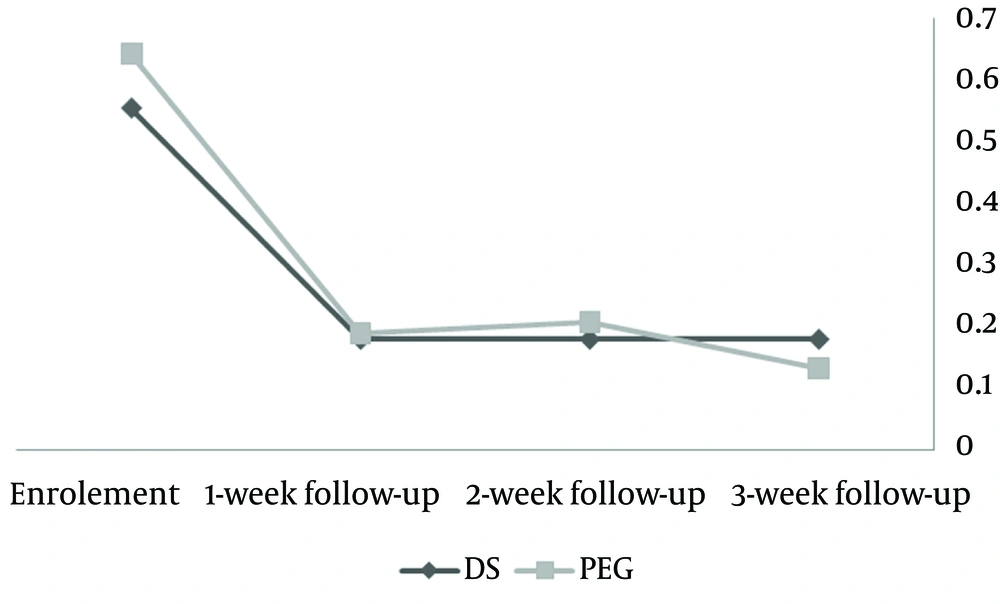
4.3. Pain and Retention
Following three weeks of medication, hard stool frequency, painful defecation and stool retention decreased in both groups. However, the differences between groups were not statistically significant (Figure 1).
4.4. Blood Stained Stool
Median blood stained stool in baseline and weeks 1, 2 and 3 were 0 for both groups. Only one patient in D. sophia group and one in the PEG group reported a history of blood stained stool at the end of the third week [0 (0 - 0) in both groups; P = 0.365].
4.5. Adverse Events
During the first three weeks of treatment and despite of the dose increasing, 10 patients (18.9%) in PEG and 3 (5.4%) in D. sophia group needed bisacodyl suppositories after seven days of no bowel movement. The difference between two groups was significant (P = 0.03).
At the beginning of the intervention, the number of patients who suffered from flatulence and abdominal pain in D. sophia group (22 = 39.3%) was more than that of the PEG group (20 = 37.7%). At the end of the eighth week, values of these parameters were less in the D. sophia group (5 = 8.9%) as compared to the PEG group (6 = 11.3%) (P = 0.461) (Table 4). The number of patients who disliked the taste of medication in the D. sophia group (17 = 30%) was significantly more than that of the PEG group (5 = 9.5%) (P = 0.004).
| Variables | DS (n = 56) | PEG (n = 53) | P value |
|---|---|---|---|
| 22 (39.3) | 20 (37.7) | 0.512 | |
| 14 (25.0) | 20 (37.7) | 0.110 | |
| 12 (21.4) | 15 (28.3) | 0.271 | |
| 10 (17.9) | 11 (20.8) | 0.444 | |
| 5 (8.9) | 6 (11.3) | 0.461 |
5. Discussion
D. sophia is a cheap and available medication which can be introduced as a safe alternative for PEG in CFC. However, the taste may not be much acceptable. The D. sophia mechanism of action has not yet been elucidated. The seeds may act as a stool softener and bowel smooth muscle relaxant. D. sophia seeds oral solution produce mucilage that can absorb water from bowel lumen and thus may soften the stool. The compound, allyl disulfide in D. sophia seed may have relaxant effect on the smooth muscles and facilitate the defecation (19). As PEG is often mentioned as the first-line treatment for CFC, it was selected in the current study. Many studies have confirmed the related effects of PEG superiorities rather than the other laxatives in the treatment of CFC.
Findings of the study conducted by Karami et al. showed the preference of PEG compared with liquid paraffin in treatment of CFC (20). Wang et al. found that two weeks treatment of CFC with PEG 4000 and lactulose is safe. But PEG revealed higher rates of success in the improvement of stool frequency than lactulose (21). In a meta-analysis, Lee-Robichaud et al. compared the efficacy of PEG and lactulose and showed the superiority of PEG over lactulose in treatment of functional constipation in both children and adults (22).
Studies showed that PEG is very effective in the control of constipation. This medicament can rapidly stop the symptoms, even in patients with fecal impaction. Oral PEG can be used as a safe alternative of enema in patients with fecal impaction (23). Savino et al. found that enema and PEG are equally effective in treatment of fecal impaction in constipated children (24).
A positive family history of constipation is found between 30 and 40 percent in different studies (24). In our study, 49 (45%) patients had positive family history of constipation. There was no relationship between positive family history and the result of treatment in both groups. A relationship between increasing body mass index and lower bowel movement frequency has been reported repeatedly in adults and children (25). Prevalence of obesity and overweight in Iranian children is 5.1% and 10.8%, respectively (26). The rates of overweight and obesity were 9.2% and 12.8% in our study.
According to previous studies, the chance of recovery with PEG in CFC of children is about 56 - 90% (8). The less chance of recovery in our study may be due to the chronicity of disease in most of the patients. Considerably, history of receiving multiple drug and tolerance to many medications are additional parameters, especially in D. sophia group. Despite this fact, the recovery rate with D. sophia was found to be higher at the end of the eighth week than that with PEG. According to the relative supremacy of D. sophia versus PEG at the end of the eighth week, D. sophia was found to be more slowly-acting (64.3% vs. 54.7%).
5.1. Strengths and Limitations
Blinding was not possible regarding the differences in the appearance and dose of the applied medications. However, the methodologist and the statistician who assessed and analyzed the data were blind to the study. Taste of D. sophia was a limitation of consumption. The number of patients in D. sophia group who disliked the taste of medicine was more than that of the PEG group. Follow-ups were satisfactory and we missed no case to follow-up after starting the treatment. Data were collected from 100% of the parents by two follow up sessions at the end of the third and eighth week.
D. sophia is a safe and cheap alternative for PEG. Efficacy of D. sophia is as much as PEG in the treatment of CFC. Compared to PEG, D. sophia showed better response in the control of annoying symptoms such as abdominal pain and flatulence. According to the outcomes of this trial, DS oral solution can be tastely modified to be applied for respective treatment in a long period. The long-term safety and efficacy of D. sophia should be evaluated by more comprehensive randomized clinical trials.