1. Background
In 2009-2010, the prevalence of obesity in children and adolescents was 16.9 % (1). Obesity in adulthood has its origins mainly in childhood. This makes obesity a pediatric concern for which the necessary therapeutic interventions should be undertaken in this period (2). Currently, the accepted definition of obesity for adults is body mass index (BMI) ≥ 30 kg/m2 (3). Children are overweight when BMI is greater than 85th percentile and obese when BMI is greater than 95th percentile (4, 5).
Obesity affects around one-fifth of children and adolescents in industrialized countries (6) and is associated with various comorbidities (7), which affect the society’s general health tremendously. The high rates of overweight among children and obesity among adults remain a major public health concern (8). Alongside comorbidities like hypertension and diabetes mellitus, which may affect kidney, great BMI is independently associated with proven risk for numerous kidney disorders such as chronic kidney disease, end stage renal disease, renal cell carcinoma and nephrolithiasis (9).
Although kidney length is interconnected to the age of the child (10), the effect of obesity on various organs, including kidney is non-negligible, as children with malnutrition are proven to have less length and volume of the kidney in previous studies (11).
Renal length is considered as a fundamental parameter in clinical assessment of kidney diseases (9) and norm grams specify renal length only for age, therefore we need established norm grams to categorize normal renal length in accordance to BMI in order to reduce unnecessary evaluations for nephromegaly. Moreover, ethnicity plays a great role in determining organ length, and various epidemiologic studies need to be conveyed to detect the normal organ length in each community.
2. Objectives
Therefore, we designed this cross-sectional cohort study in an Iranian sample to ignite the idea to form norm grams of kidney length related to BMI.
3. Patients and Methods
3.1. Patient Selection
hundred children and adolescents, aged 1 - 19 years, referred to the pediatric clinics of three hospitals (Rasoul-e-Akram and Shahid Fahmideh) in Tehran between June 2010 and 2012, were enrolled into the study after the nephrologist’s and endocrinologist’s approval. Fifty obese children and adolescents were selected as the case group, who were matched by age and gender to 50 non-obese children and adolescents as the control group. Participants were allocated according to the 2000 CDC growth charts (5); normal weight was defined as BMI = 5-85th, and overweight/obese as BMI ≥ 95th percentile. Participants between 85th and 95th percentiles were not enrolled in the study. Any patient with abnormal blood pressure, family or personal history of any urinary diseases, obesity due to any systemic disease was excluded from the study. All participants underwent abdominal-pelvic ultrasonography, which was done by a single specialist, using a 3.5 MHz sector probe with an accuracy of 0.1 mm. Each kidney was measured separately and the largest longitudinal dimension was measured in deep inspiration. Any patient with abnormal renal appearance on ultrasound was excluded from the study. Data were collected for each patient including age, gender, height, BMI and body surface area (BSA). The protocol of the survey has been proven by the ethic committee of Iran University of Medical Sciences, Tehran, by the code 89-04-140-12348.
3.2. Statistical Methods
Statistical analysis was performed by SPSS 11.5 for windows. Analysis of the data distribution was assessed by the Kolmogorove-Smirnove test. Mean and standard deviations or median with interquartile range were calculated for quantitative data and frequency with percent for qualitative data. For determining the relationship between kidney length and all independent variables, the Pearson coefficient of correlation was used. Predictor factors for kidney length were determined by a forward multiple linear regression analysis for the right and left kidney differences. The kidney length between groups was compared by Independent-samples t-test or its non-parametric test, Mann-Whitney test; paired-sample t test was used to compare left and right kidney length within the groups. Less than 0.05 was accepted as indicating statistical significance.
4. Results
We included 50 obese children and adolescents (case group) and 50 normal weights (control group). The mean age of case group was 134.62 ± 47.97 (range 45-210, median 138) months and of the control group (range 30 - 207, median 136.5) months. The case group had similar mean age (P = 0.838), gender (P = 0.547) and age group (P = 0.807) to controls, but as expected higher weight, BMI and BSA than that of control group (P < 0.001 for all) (Table 1). There was no significant difference of mean height in groups after we had adjusted it for age (P > 0.05). There was no significant difference between male and female case group in age, weight, height, BMI, BSA (P > 0.05) (Data are not shown). Clinical characteristics of both groups are shown in Table 1. Paired Student’s t-test demonstrated that length of the left kidney was significantly larger than that of the right kidney in obese and control groups (P < 0.001 for both groups; Table 2).
| Variable | Control Group | Obese Group | P Value |
|---|---|---|---|
| 0.547 | |||
| Male | 27 (54) | 24 (48) | |
| Female | 23 (46) | 26 (52) | |
| 132.62 ± 49.40 | 134.62 ± 47.97 | 0.838 | |
| 144.44 ± 23.0 | 145.94 ± 22.05 | 0.744 | |
| 39.20 ± 15.96 | 67.02 ± 24.83 | ||
| 17.74 ± 2.21 | 29.54 ± 5.14 | ||
| 0.10 ± 0.53 | 2.37 ± 0.44 | ||
| 1.23 ± 0.36 | 1.63 ± 0.43 | ||
| 0.807 | |||
| 1-72, mon | 7 (14) | 5 (10) | |
| 6-12, y | 20 (40) | 22 (44) | |
| 12-19, y | 23 (46) | 23 (46) |
Clinical Characteristics of Obese Group and Controla
| Age Ranges Groups | Control Group | Obese Group | P Value |
|---|---|---|---|
| Left kidney length | 66.14 ± 10.94 | 68.80 ± 8.79 | 0.664 |
| Right kidney length | 64.86 ± 10.06 | 66.00 ± 7.71 | 0.836 |
| Left kidney length | 83.80 ± 4.59 | 88.45 (85.00-94.25) | 0.004 |
| Right kidney length | 82.85 ± 4.50 | 86.73 (85.75-91.25) | 0.003 |
| Left kidney length | 99.61 ± 6.15 | 100.50 (98.00-100.80) | 0.019 |
| Right kidney length | 96.52 ± 5.78 | 100.65 ± 4.99 | 0.013 |
| Left kidney length | 88.20 ± 13.36 | 93.60 ± 12.50 | 0.040 |
| Right kidney length | 86.22 ± 12.47 | 91.26 ± 12.20 | 0.044 |
Left and Right Kidney Length Between Control and Obese Group in Three Age Rangesa
Also analysis revealed that left and right kidney length in obese group was significantly higher than in control group (P = 0.044 and 0.040, respectively); after dividing two groups into three age ranges, the same result was found for 6, 12, 14 year and also 12-19 year ranges (Table 2); the difference of kidney length between the two groups was still found significant while controlling the effect of age, height and sex (P < 0.001). We found significant gender-related difference in right kidney length in neither the control (86.26 ± 13.20, male vs. 86.17 ± 11.85, female; P = 0.981) nor obese (90.92 ± 10.86, male vs. 91.58 ± 13.52, female; P = 0.851), the same result was found for the left kidney length, in the control (89.26 ± 13.78, male vs. 86.96 ± 13.04, female; P = 0.549) and the obese group (94.33 ± 10.98, male vs. 92.92 ± 13.94, female; P = 0.695).
Analyzing data yielded a significant linear association between right and left kidney length with each other and with age, height, weight, BMI and BSA (P < 0.0001 for all) in control and obese group (Table 3). In the obese group, the correlation coefficients for right kidney length with all these independent variables were similar to that for the left kidney length; a similar result was found for the control group; as shown in Table 3, all these coefficients for the control group are higher than those in obese group.
| Predictor Model | Equation | R2 | P Value |
|---|---|---|---|
| < 0.001 | |||
| Obesity statues, height, age | 35.170 + 4.440x + 0.245y +0.119z | 0.835 | |
| Obesity status, height | 15.863 + 4.309x + 0.487y | 0.820 | |
| <0 .001 | |||
| Obesity statues, height, age | 40.773 + 4.805x + 0.177y + 0.165z | 0.832 | |
| Obesity statues, height | 13.858 + 4.628x + 0.515y | 0.859 |
Multiple Linear Regression Equation of of Obese and Control Groups For Right and Left Kidneysa
Linear regression equations for the length of kidneys and independent predictors such as obesity status, age and height are shown in Table 4. These variables have a significant positive relationship with the length of kidneys; on the other hand, with increasing height (right kidney length: β = 0.245; left kidney length: β = 0.177) and age (right kidney length: β = 0.119; left kidney length: β = 0.165), the average length of kidneys increases. Also according to the β coefficient for the obesity status in the equations for right (β = 4.440 mm) and left (β = 4.805 mm), kidney lengths for the obese children and adolescents were significantly higher than those for the normal weight group with the same age and height (adjusted for age and height). When age was removed from the model, obesity status and height still remained significant predictors of kidney length (P < 0.001; Table 4).
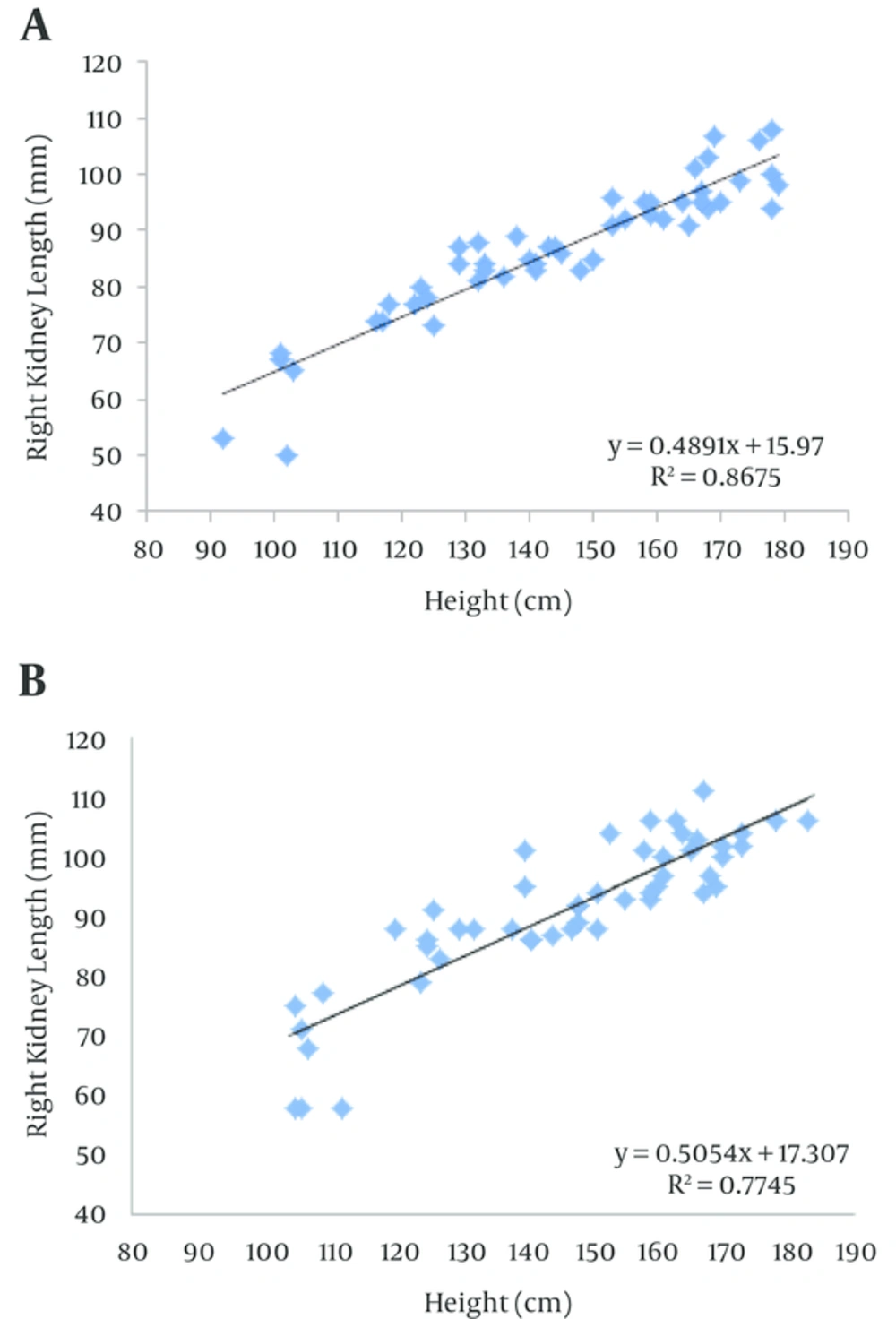
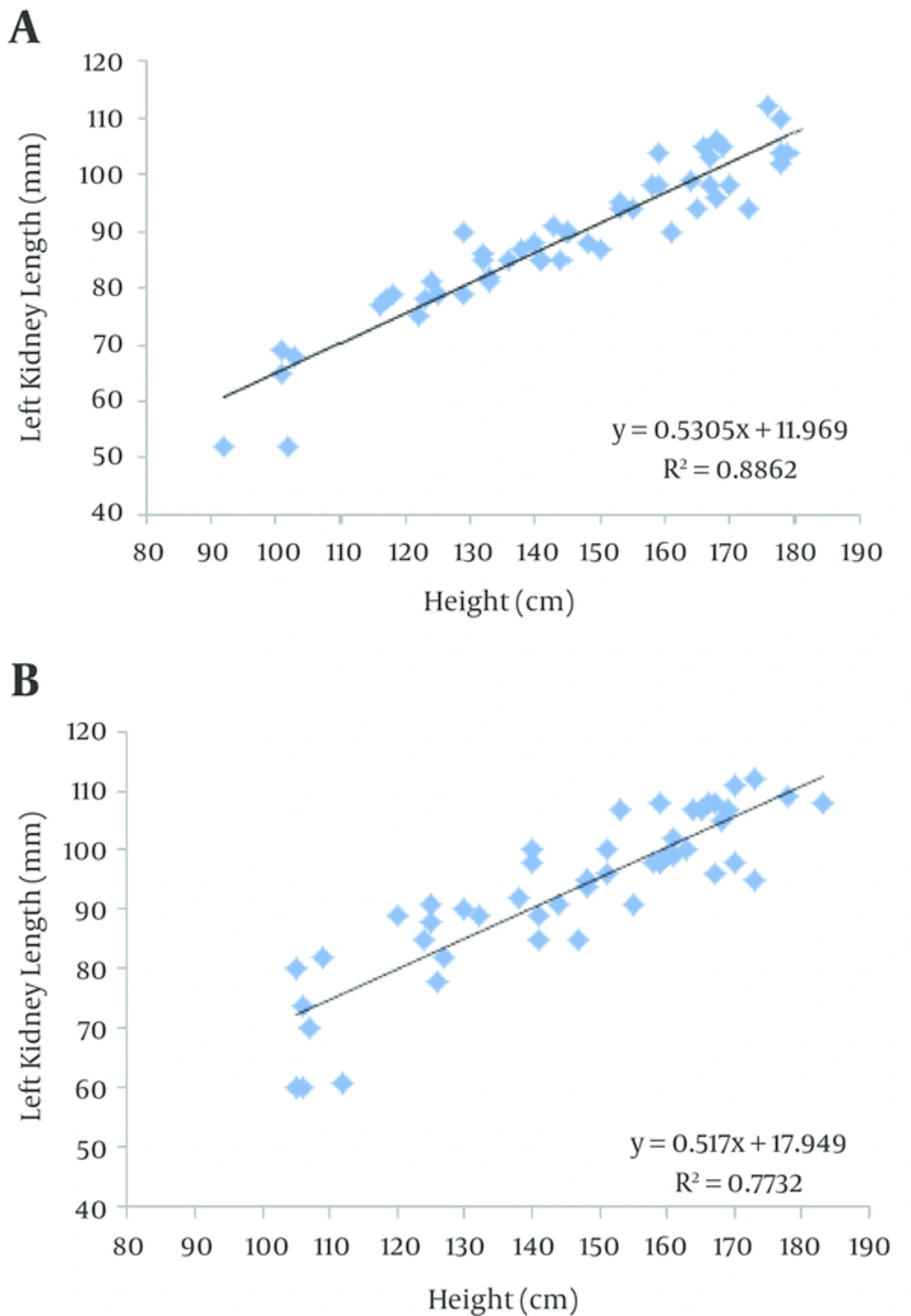
Linear regression equations for right and left kidney lengths and height were then created for both groups (Figures 1 and 2). The scatter plots illustrate that the height is linearly related to length of both kidneys in obese and control groups. The equations for the obese and control groups were found to parallel each other and were also significantly different from each other for both right and left kidneys (P < 0.01).
| Parameter | Age, mon | Height, cm | Weight, Kg | BMI, kg/m2 | BSA, cm2 | Left Kidney |
|---|---|---|---|---|---|---|
| 0.971 | ||||||
| Left kidney length | 0.949 | 0.935 | 0.914 | 0.815 | 0.938 | |
| Right kidney length | 0.931 | 0.924 | 0.895 | 0.796 | 0.923 | |
| 0.941 | ||||||
| Left kidney length | 0.893 | 0.881 | 0.864 | 0.564 | 0.882 | |
| Right kidney length | 0.876 | 0.882 | 0.876 | 0.634 | 0.891 |
Pearson Correlation Between Kidney Length and Age, Height, Weight, BMI and BSA For Both Groupsa
5. Discussion
Our results showed that left and right kidney length in obese group was significantly higher than in control group, the same result was found in 6-12 year and also 12-19 year ranges and it was not seen for first range (1-72 month range); that is because the sample size in 1-72 month group is too small to show such a difference. Our study demonstrated that in both groups the kidney length increases with age, height and BMI, and also linear regression equations formulated for right and left kidney may help physicians reconsider the cut-off level of kidney size for obese patients to inhibit the flaw of investigating these children and adolescents for organomegaly.
Many studies have investigated the direct and indirect effects of obesity on kidney diseases, such as end stage renal disease and chronic kidney disease (6, 10, 11) and some other have justified that solving obesity will solve these diseases (11). Others have concluded that malnutrition is also associated with lower kidney size (12). Yet, these studies have the confounding factor of including patients with co-morbidities and therefore cannot evaluate the pure effect of obesity on kidney size.
In the current study we excluded all children and adolescents with any kidney abnormality in order to assess the pure effect of obesity on the kidney length and have included children and adolescents of all ages. Some studies have studied children under two years; Akhavan et al. have proposed an age-based formula for predicting renal length in children aged 0-18 months (9). Schmidt et al. have also reported variations according to age, height and body composition of 717 healthy children of 0-18 months (13). Geelhoed et al. have also reported some variations associated with infantile and maternal factors (14). These studies mostly emphasize the characteristic changes of kidney volume in children and adolescents younger than two years and therefore cannot be generalized to all children.
These studies have associated larger kidney size in males to sex steroid and growth hormone in addition to body composition. Our study considered children and adolescents of all ages and found no significant difference in kidney length regarding gender neither in case nor in control group. Some studies have assessed kidney weight in autopsies, which was best predicted by body surface area and was lower in obese (15). Zuzuarregui et al. have conducted a retrospective study on 204 healthy children in 2009 and proposed a linear norm gram for kidney size based on height, using ultrasonography, and have reported that healthy obese children have enlarged kidneys with no abnormality, but have declared some complications in norm gram, as they have included pure hematuria (16). Yet we have divided the participants into two groups regarding their BMI in order to be able to assess the pure effect of obesity on kidney length and have excluded all abnormalities, even pure hematuria, in order to be able to assess healthy kidneys. On the other hand, each society might have different values, regarding ethnicity, race and etc. discrepancies.
Parallel to the Zuzuarregui’s study, we have also found no gender-related difference in kidney length. The height of the obese group has been proposed to be larger in some studies (16), but we did not find any difference in height of the two groups.
These results urge a re-consideration before marking the obese child with nephromegaly, in order to prevent unnecessary clinical assessments and save patient and physician’s time, money and energy.
Further studies might imply that the definition of organomelies ought to change in obese children and adolescents and norm grams, e.g. CDC norm grams, need to be validated for assessing organ lengths according to the effective predictors, such as age, height and BMI. Our study had some limitations. First, we only measured the kidney length by ultrasound, but the kidney volume might be more appropriate for a better assessment of kidney. Second, this study could be done with a larger sample size to reduce the probable bias occurring, although we have seen positive effect with this sample size too. Considering the difference in kidney size of obese children and adolescents compared to leans and the growing prevalence of obesity in our society, it is worthwhile to conduct a large cohort study on all societies to formulate a specific standard cut-point limit or norm gram for obese children and adolescents, in order to facilitate the diagnosis of kidney diseases, including organomegaly, in obese children and adolescents.
In conclusion we can say that in all age groups of children and adolescents, there is a significant difference in kidney length between normal weight and obese participants and interpretation of nephromegaly for obese children and adolescents can lead to some unnecessary workups, when decided according to age-specified charts, which have not taken BMI into account.