1. Background
Despite substantial improvements in modern pediatric dentistry, a number of children cannot cope with dental treatment by behavior guidance techniques. In these patients pharmacological intervention may be advocated (1, 2) to replace the previous experiments with a different new treatment approach that is known as retraining process (3). Among different categories of sedative agents, benzodiazepines have been prescribed for more than three decades to patients of all ages. The effective and predictable sedative and amnestic effects of benzodiazepines support their use in pediatric patients. Midazolam is one of the most extensively used benzodiazepines in this age group. Oral form of drug is the best accepted route of administration in children (3-7). However commercially prepared midazolam syrup is not available in some countries. In addition the cost of the drug is relatively high, that is another concern in daily practice. As an alternative a mixture of IV injectable midazolam and a flavoring agent has been used (8). The flavoring vehicle is used to mask the bitter taste and to adjust the pH of the drug (9, 10), that is an important consideration in drug absorption (11). Since the midazolam syrup is not accessible in our country, and due to lack of similar studies, the main objective of this investigation was to compare the efficacy and safety of an extemporaneous form of drug that is prepared by pharmacist (orally administered IV midazolam) and a commercially midazolam syrup.
In addition, it is shown that not every child benefits from midazolam sedation. There are a variety of factors other than the type of sedative and its dose that involve in effectiveness of midazolam. The effects of child’s temperament and behavioral problem on midazolam effectiveness are not investigated extensively. Fear/anxiety and behavioral problems (2, 12), and high levels of impulsivity (13) and emotionality (14) are among factors that may contraindicate midazolam premedication in children. Determining those children who do not benefit from sedation is important to clinicians like pediatric dentists who encounter uncooperative children in a daily basis.
2. Objectives
The main objective of this investigation was to compare the efficacy and safety of an extemporaneous form of drug that is prepared by pharmacist (orally administered I.V midazolam) and a commercially midazolam syrup. As an adjunct objective the present study investigated whether differences concerning sedation success can be explained by child’s behavioral problems and dental fear.
3. Patients and Methods
3.1. Samples
After gaining written parental consent, eighty eight uncooperative pediatric patients aged 3 to 6 years, who were referred to Pediatric Clinic of Guilan Dental School for uncooperative behavior participated in this double blind and randomized controlled clinical trial. The study had a two arm parallel design. Patients were randomly assigned to either midazolam syrup group or “orally administered IV midazolam” group via 6 quadric blocks. Sample size was calculated using a previous sedation study (8). Power was set at 80%, α = 0.05 and a 25% clinical difference between groups was estimated for sample size determination. All children were healthy (ASA class I) and rated as class 1 or 2 on the Frankl Behavioral Rating Scale, which means negative or definitely negative behavior (3). Patients were excluded from the study in the case of physical or mental disabilities, history of common cold in the past two weeks, Tonsil/adenoid hypertrophy (Brodsky tonsil score > 2), or any anatomical deformities in face and neck such as micrognathia and macroglossia.
3.2. Drug Regimen
Regimen A: Midazolam Maleate syrup sugar free liquid (Amsed 2.5 mg/mL UK). Commercially available syrup (Amsed, UK) included sugar free midazolam maleate syrup and was prepared in pH 4.
Regimen B: Orally administered IV midazolam 2.5 mg/mL that consisted of the injectable solution of midazolam hydrochloride (Amp. 15 mg/3 mL Midamax, Tehran Chimi, Iran,), in combination with Syrup BP (66.7% sucrose, 33.3% water as solvent) and orange extract flavoring. The compound was prepared in pH 3.5 by the responsible pharmacist with shelf life of 14 days. Adjustment to a pH of about 3.5 ensured that the drug stays stable in solution (14, 15).
Drugs were calculated by responsible researcher and administrated by dental nurse who was unaware of the study design, using a needleless syringe in the dose of 0.2 or 0.5 mg/kg. A dose of 0.2 mg/kg was administered to children over age five years and in patients less than five years of age 0.5 mg/kg was prescribed (16). The midazolam dose is routinely individualized based on patient's age (older children need less of drug), degree of anxiety, and the level of sedation desired (6). After administration of medication the child was kept under supervision for 30 minutes and then transferred to dental chair and dental treatment began. Parents were not present during dental treatment session. The dental appointment included injection and restorative/pulp treatment and did not exceed 30 minutes. All treatments were provided by a pediatric dentist who was unaware of drug regimen.
3.3. Efficacy
Sedation efficacy was rated by an examiner who was blind to the sedative regimen. Two separate criteria were used to assess sedation success: “North Carolina Behavior Rating Scale” (NC) (Table 1) and “Houpt Sedation Rating Scale” (Table 2) (17). The NC (18) was used to evaluate the child’s behavior at the moment of local anesthesia injection, and at cavity preparation. The other evaluation criterion “Houpt Sedation Rating Scale” (17) divides the sedation status to four subdomains of sleep, movement, crying and overall behavior (Table 2).
| Behavior | Definition |
|---|---|
| Patient is quiet or sleeping with only extraneous, inconsequential movements | |
| Patient is cooperative for treatment but with 1 or 2 undesirable behaviors | |
| Patient noticeably disturbed, with 2 to 3 undesirable behaviors | |
| Patient extremely defiant with presence of all undesirable behaviors |
Definition of North Carolina Behavior Rating Scale a
| Rating Scale | Definition | Score |
|---|---|---|
| Fully awake, alert | 1 | |
| Drowsy, disoriented | 2 | |
| Asleep | 3 | |
| Violent movement that interrupts treatment | 1 | |
| Continuous movement that makes treatment difficult | 2 | |
| Controllable movement that does not interfere with treatment | 3 | |
| No movement | 4 | |
| Hysterical crying that interrupts treatment | 1 | |
| Continuous, persistent crying that makes treatment difficult | 2 | |
| Intermittent, mild crying that does not interfere with treatment | 3 | |
| No crying | 4 | |
| Aborted | No treatment | 1 |
| Poor | Treatment interrupted, only partial treatment completed | 2 |
| Fair | Treatment interrupted but eventually all completed | 3 |
| Good | Difficult, but all treatment performed | 4 |
| Very good | Some limited crying or movement, e.g. during anesthesia or mouth prop | 5 |
| Excellent | No crying or movement | 6 |
Definition of Houpt Sedation Rating Scale a
3.4. Safety
Safety of the two sedative regimens was evaluated by physiological parameters including Heart Rate (HR), Respiratory Rate (RR), oxygen saturation (SpO2) and blood pressure (BP). All the patients were monitored using a pulse oximeter (SANOFI A 320, China) and digital blood pressure device. Oxygen saturation was constantly monitored. Hypoxemia was determined as SpO2 less than 93% (8). Physiological parameters were rated before sedation as baseline measure, and then at the beginning of treatment and every 15 minutes until the end of treatment.
3.5. Behavior Problems and Dental Fear
The second objective of the present study was to determine the success rate of sedation in children with and without behavior problems among study population. An assessment of child’s behavioral problems, was conducted using the “Strengths and Difficulties Questionnaire (SDQ)” (20). The SDQ is a brief mental health questionnaire for children and adolescents. It can screen the behavioral problems (by total difficulty score) and the emotional sub-scale can show the general anxiety of the children. The 25 SDQ items are divided into five scales. These scales are SDQ 1/conduct problems, SDQ 2/hyperactivity-inattention, SDQ 3/emotional symptoms, SDQ 4/peer problems and SDQ 5/pro-social behaviors.
The mentioned scales scores (except the last one) are added up to generate a total score. The reliability and validity of Persian version of questionnaire has shown in Iranian population by Tehranidoost et al. (20). Scoring and interpretation of scores were done by the institution of cognitive sciences.
As the other measure we assessed the level of child’s dental fear; to determine the sedation success in children with and without dental fear using Child Fear Survey Schedule-Dental Subscale (CFSS-DS) questionnaire. This Likert type questionnaire includes 15 questions to be completed by parents. The patients score can be placed in the range of 15 - 75 and > 38 is considered as dental fear (21). Data were statistically analyzed using the Independent t-test and Chi-Square test. Significance level was established at P < 0.05 in SPSS16 software environment.
4. Results
A total of eighty eight children participated in the study (45.5% males and 54.5% females). The mean age of patients in group A (midazolam syrup) and B (orally administered IV midazolam) was 72.2 ± 11.9 and 68 ± 12.4 months respectively. The mean weight of children was 20.2 ± 5.4 and 18.4 ± 3.9 kg in groups A and B respectively. There were no statistical differences in gender, age and weight between the two groups. The flow diagram of the participants is presented in Figure 1.
4.1. Sedation Assessment
Behavior at the time of injection and cavity preparation (North Carolina scale): Ratings of North Carolina scale are summarized in Table 3. The participants of the two groups did not show significant difference in their behavior during injection and cavity preparation. Quiet and annoyed behaviors were the most observed behaviors. Wild behavior was the least observed behavior in both groups.
| Group | Quiet | Annoyed | Upset | Wild | P Value |
|---|---|---|---|---|---|
| 0.5 | |||||
| A | 17 (38.6) | 20 (45.5) | 7 (15.9) | 0 (0) | |
| B | 19 (43.2) | 17 (38.6) | 6 (13.6) | 2 (4.5) | |
| 0.4 | |||||
| A | 24 (54.5) | 14 (31.8) | 6 (13.6) | 0 (0) | |
| B | 19 (43.2) | 17 (38.6) | 6 (13.6) | 2 (4.5) |
Frequency of Exhibited Behavior During Sedation by North Carolina Behavior Rating Scale a
Level of sleep, crying, movement and overall behavior (Houpt): Houpt Behavior Rating Scale was used to determine level of sleep, crying, movement and overall child’s behavior during each sedation appointment (Table 4). There was not significantly different between groups. In both groups almost all patients were alert/drowsy. Hysterical crying, violent movement and aborted treatment was observed in one case in group B.
| Domain | A | B | P Value |
|---|---|---|---|
| 0.2 | |||
| Alert | 20 (45.5) | 26 (59.1) | |
| Drowsy | 22 (50) | 18 (40.9) | |
| Asleep | 2 (4.5) | 0 (0) | |
| 0.6 | |||
| Hysterical | 0 (0) | 1 (2.3) | |
| Continuous | 7 (15.9) | 8 (18.2) | |
| Intermittent | 17 (38.6) | 19 (43.2) | |
| No crying | 20 (45.5) | 16 (36.4) | |
| 0.6 | |||
| Violent | 0 (0) | 1 (2.3) | |
| Continuous | 11 (25) | 10 (22.7) | |
| Controllable | 12 (27.3) | 15 (34.1) | |
| No movement | 21 (47.7) | 18 (40.9) | |
| 0.6 | |||
| Aborted | 0 (0) | 1 (2.3) | |
| Poor | 1 (2.3) | 0 (0) | |
| Fair | 3 (6.8) | 5 (11.4) | |
| Good | 8 (18.2) | 7 (15.9) | |
| Very good | 19 (43.2) | 22 (50) | |
| Excellent | 13 (29.5) | 9 (20.5) | |
| Total | 44 (100) | 44 (100) |
Houpt Sedation Rating Scale in the Two Groups
For a better perception of sedation outcome the overall behavior was dichotomized into acceptable (excellent, very good and good) and unacceptable (fair, poor and aborted) behavior.
The acceptable behavior was observed in 90.9% of patients in group A (CL: 0.85 - 0.96) and in 86.4% of group B (CL: 0.87 - 0.93). The mean age of patients with overall acceptable and unacceptable behavior was compared. There was no significant difference between the mean age of children with each category of behavior (Table 5).
| Behavior of Groups | n | Mean Age, mo | P Value |
|---|---|---|---|
| Acceptable | 40 | 71.6 | 0.2 |
| Unacceptable | 4 | 79.7 | |
| Acceptable | 38 | 68.1 | 0.9 |
| Unacceptable | 6 | 67.8 |
Mean Age of Children With Acceptable and Unacceptable Behavior in the Two Groups
4.2. Physiologic Parameters
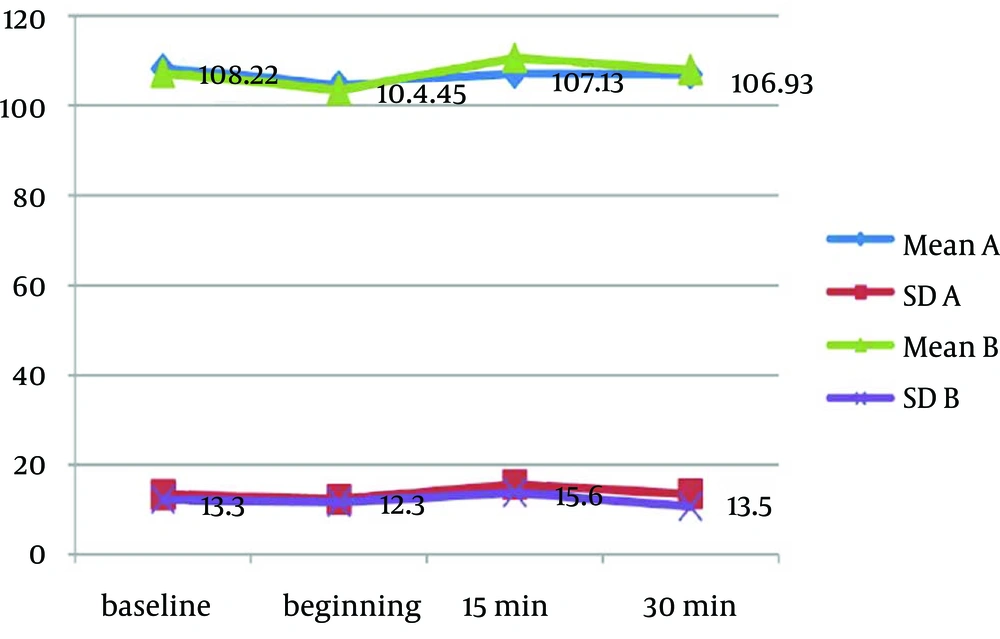
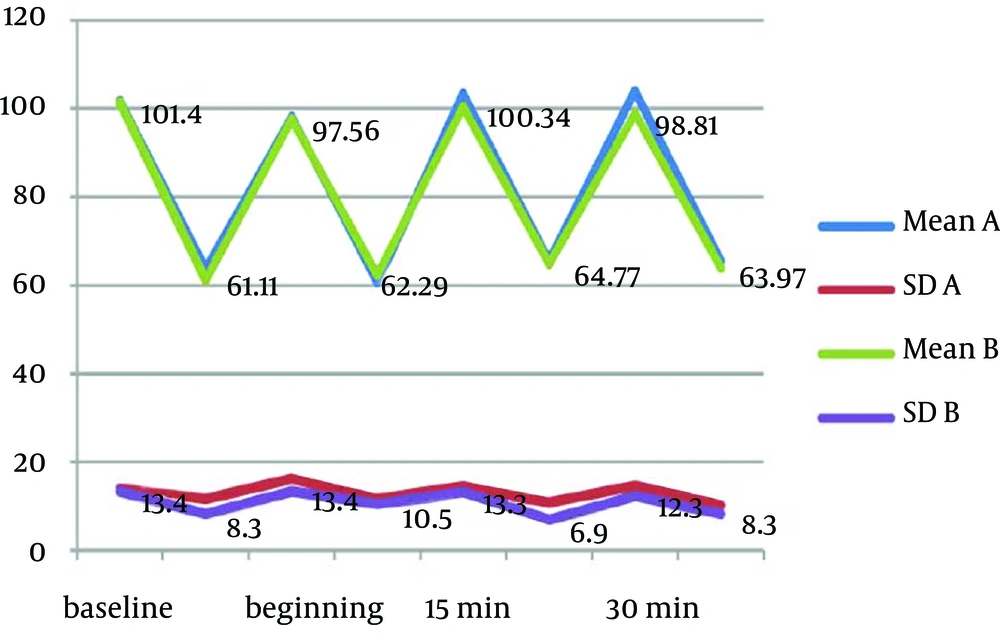
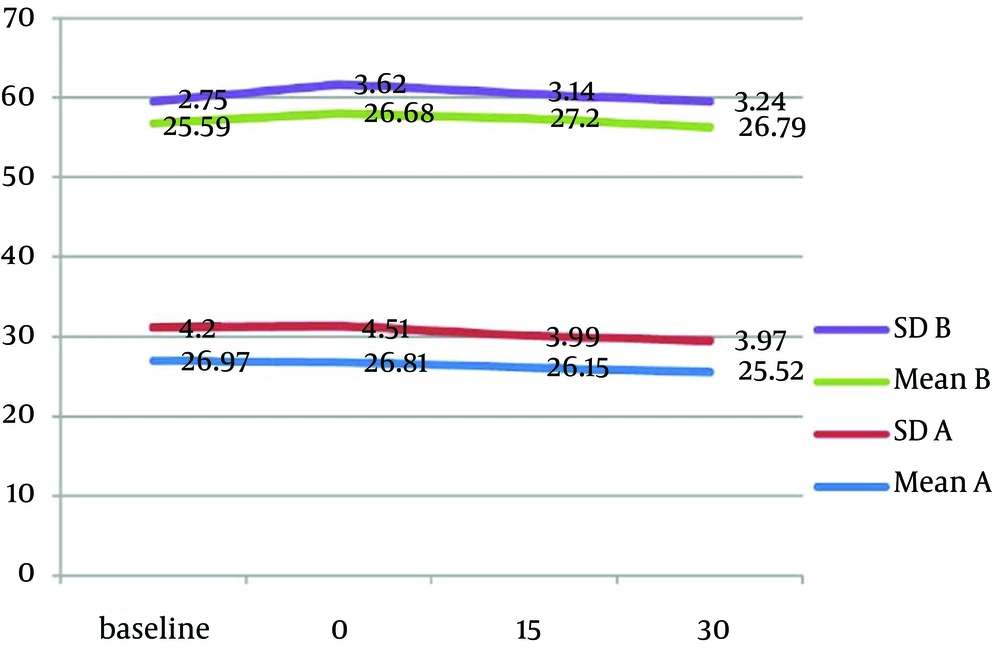
Mean values of physiological parameters (SpO2, HR, BP, and RR) are summarized in Table 6 and Figure 2 to 4. There were no significant differences in any of physiological parameters during the sedation sessions between the two groups except for SpO2 level at baseline and at the beginning of treatment that was higher in group A (Table 6). Figures 1 to 3 demonstrate the mean values of heart rate, blood pressure and respiratory rate of the two study groups. Serious adverse effects were not observed during and after sedation appointment except for one case of vomiting immediately after drug administration.
| SpO2 | Group | Mean (SD) | P Value |
|---|---|---|---|
| A | 97.5 (1.7) | 0.02 | |
| B | 98.2 (1.1) | ||
| A | 97.1 (2.1) | 0.01 | |
| B | 98.0 (0.7) | ||
| A | 97.5 (1.8) | 0.1 | |
| B | 98.0 (1.3) | ||
| A | 97.6 (1.7) | 0.09 | |
| B | 98.1 (0.7) |
Mean Oxygen Saturation Data in Each Group
4.3. Behavior Problems (SDQ) and Dental Fear (CFSS-DS)
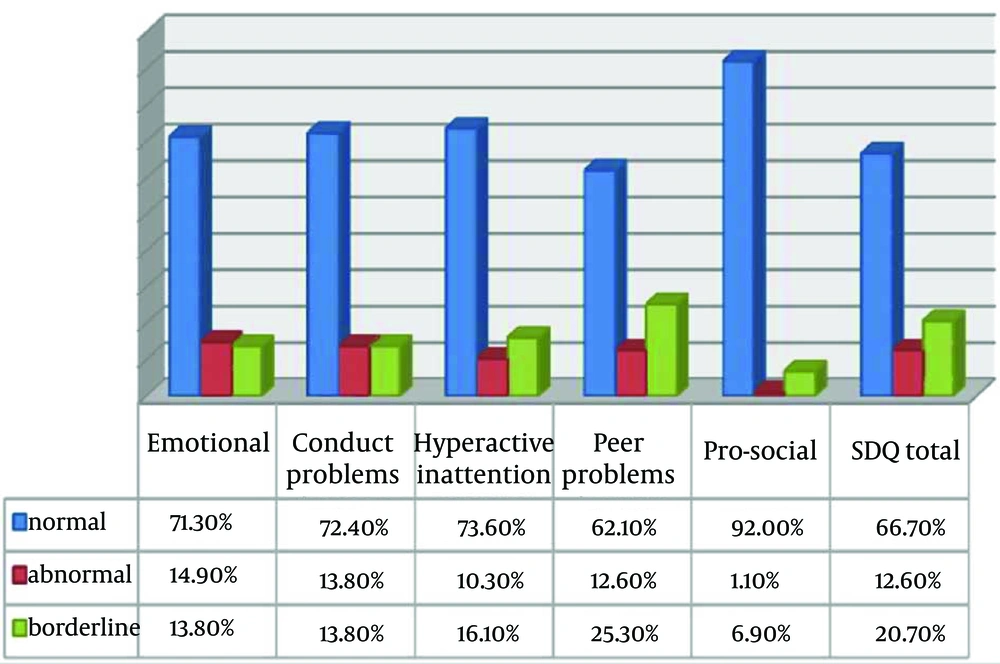
From eighty eight patients 12.6% revealed symptoms of behavioral problems using Strength and Difficulties Questionnaire (SDQ). The distribution of Total SDQ and its subdomains in study population is presented in Figure 5. There was no significant difference between overall behaviors among three categories of behavioral problems (normal, abnormal, and borderline), Chi-square P = 0.7. From all study population 89.7% of children without signs of behavioral problems, 88.9% of borderlines and 81.8% of behavioral problems demonstrated acceptable behavior during treatment. Dental fear was observed in 50.4% of children. Out of fifty one dentally fearful patients, 84.3% demonstrated acceptable behavior at sedation session. Chi-square test P = 0.1.
5. Discussion
This double blinded, parallel randomized and controlled trial at first step attempted to evaluate and compare the efficacy and safety of midazolam syrup versus orally administered IV midazolam. Our results demonstrated that both regimens were safe with desirable sedation effects in uncooperative pediatric patients. We did not found any similar study that compares these two formulations; however injectable midazolam-Syrpalta combination showed to be more effective than commercially available syrup by Brosius (22).
There are also a number of studies that investigated different doses of “orally administered IV midazolam” with each other or compared it with placebo (22-24).
The safety of preparation was evaluated by physiological parameters. No significant differences were found between the two preparations in any of physiological parameters for the overall sedation time. Although group A had a significantly lower SpO2 at baseline and at the beginning of treatment, it was over 97% and had no clinical importance. No episodes of desaturation were observed during sedation time except momentary changes during crying. These episodes of decreased SpO2 never declined under 92%.
The other physiological parameters remained in normal limit during the sedation appointment. Physiological parameters were not recorded specifically at the times of injection, cavity preparation or crying, however this variable was under the control and monitored continuously to avoid any untoward effect.
Another consideration in sedation is to determine those children who benefit from midazolam sedation. The results showed that the prevalence of acceptable overall sedation ratings in children with and without symptoms of behavioral problems using SDQ test was similar. However, due to little number of children with behavioral problems these results should be interpreted with caution. Lack of commercially available oral liquid formulations poses a frequent challenge in providing medications for patients with special needs such as pediatric patients, geriatric patients, and patients with feeding tubes to meet the needs of these patients (25). This study demonstrates promising results by pharmacist-made syrup that will make treatment of uncooperative patients more feasible. Adjusting the pH of product prevents inconsistencies in drug bioavailability. A limitation of this preparation was its short shelf life (14 days); increased stability until 102 days have been reported (26).
Under the conditions of this study we conclude that “orally administered IV midazolam” in a dose of 0.2 - 0.5 mg/kg provides safe and effective sedation in 3 to 6 years old uncooperative children. Additionally, our study demonstrated that majority of patients with symptoms of behavioral problems respond to midazolam in an appropriate manner. More research is needed either by increasing the dose or using alternative agents in children with unsuccessful midazolam sedation.