1. Background
Hearing impairment in infants is a particularly serious obstacle to their optimal development and education, including language acquisition. According to a range of studies and surveys conducted in different countries, around 0.5 - 6 in every 1,000 neonates and infants have congenital or early childhood onset sensorineural deafness or severe-to-profound hearing impairment (1). In Iran, the prevalence of hearing loss is 5 in 1,000 live births on average (2). Deaf and hearing-impaired children often experience delayed development of speech, language and cognitive skills, which may result in slow learning and difficulty progressing in school (1).
There is scientific evidence to suggest that early identification (three-six months) and administration of appropriate intervention at or before six months of age provides children with impaired hearing with the opportunity to develop normal speech and language. As a result, many countries have implemented neonatal hearing screening programs (3-10). The rationale for implementing a universal neonatal hearing screening programs is that it can detect more deaf infants, providing a greater opportunity for them to experience normal language development, while providing overall benefits in terms of reducing the disability and improving the health and well-being of the children (11).
There are two main screening interventions generally available to a number of healthcare systems worldwide. These interventions are based on electrophysiological methods: Otoacoustic emissions (OAE), and automated auditory brainstem response (AABR) (1). Both AABR and AOAE are non-invasive, rapid screening tests. OAE measures sounds that are produced by the cochlea to response to acoustic stimulation, and AABR measures electroencephalographic waveforms in response to clicks (12-15).
Factors such as limited funding, workforce shortage and the inadequate provision of follow-up and support services have prevented the implementation of the neonatal hearing screening program in the vast majority of developing countries (16).
Kemper et al. (17) conducted a survey entitled “A cost-Effectiveness Analysis of Newborn Hearing Screening Strategies.” The main objective of their study was to compare the two screening strategies: Universal screening, and targeted screening. In this two-stage procedure, OEA and AABR were the applied devices, respectively. However, in this research, the main objective was to compare AABR and OAE devices for implementing universal newborn hearing screening under a one-stage procedure. In Iran, hearing screening is conducted by implementing universal strategy, and OAE is the most applied device. Hence, this study aimed to compare the cost-effectiveness of this device and that of AABR in performing universal newborn hearing screening. We aimed to find why OAE is still the most applied device in conducting UNHS when AABR is apparently more accurate and cost-effective in the long run.
During the last decade, the rapid expansion of universal neonatal hearing screening (UNHS) programs has brought into focus questions about the most appropriate screening technology for this indication. The high prevalence of hearing loss, its subsequent burden on the health system, and the ethical issues surrounding its delayed diagnosis have necessitated the implementation of UNHS programs. However, due to the limited resources of the health system, and the possible associated outcomes and costs that these devices may have, we sought to perform a cost-effectiveness analysis (CEA), as each of these devices may have extra benefits for the UNHS program. Eventually, it may be used as a tool for evidence-informed policy-making in the field of UNHS in Iran, and for optimizing resources to control hearing loss and its resultant burden. To our knowledge, this was the first formal study to focus on the economic evaluation of screening programs for hearing impairment in Iranian newborns.
2. Objectives
The high prevalence of hearing loss and its subsequent burden on the health system and the ethical issues surrounding its delayed diagnosis have necessitated the implementation of neonatal hearing UNHS programs. However, due to the limited resources of the health system, and the possible associated outcomes and costs that these devices may have, we sought to perform a CEA, as each of these devices may have extra benefits for the UNHS program. The main objective of this study was to examine the cost-effectiveness of AABR and OAE in UNHS programs. Furthermore, it may be used as a tool for evidence-informed policy-making in the field of UNHS in Iran, and for optimizing resources to control hearing loss and its subsequent burden.
3. Methods
We applied a decision tree model with a time horizon of one year to economically evaluate the AABR and OAE devices used in UNHS. Our perspective was the health care system, and we only considered the direct costs. We defined effectiveness as the number of neonates with hearing loss, whose hearing status has been correctly detected upon using either of the devices.
In general, the cost-effectiveness of these two devices was analyzed based on the annual birth rate statistics. The diagnostic accuracy of the two devices was derived from an up-to-date and high quality research (i.e., Heidari et al.’s systematic review and meta-analysis in 2016), and newborn screening and definite diagnosis costs were derived from hearing screening centers in Iran. In other words, this study is not a primary research (like a cohort); rather it is considered as a secondary study.
3.1. Rationale of the Model
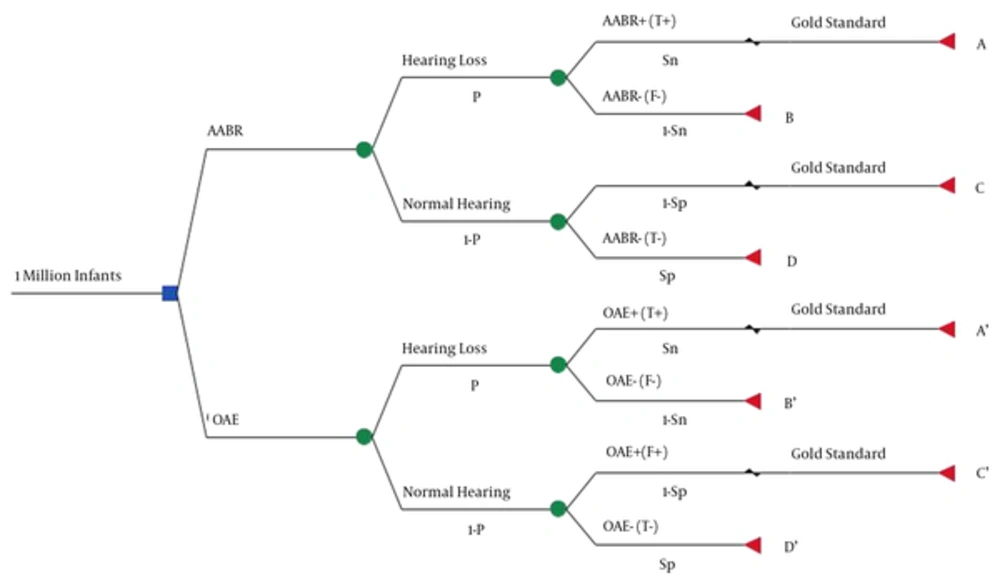
In this model, we assumed a one-million-cohort population of neonates, who were screened during the first 24 hours of birth, using one of the AABR or OAE devices in a single stage, and without loss to follow up (decision node).
These devices identify the screened neonates as normal or abnormal. This detection may be true or false, and its possibility depends on the prevalence of the hearing loss, and the sensitivity and specificity of the devices. Here, hearing loss was defined as permanent congenital bilateral hearing loss exceeding 35 dB, presuming that the screening has been performed by an audiologist. Therefore, no error occurs due to the operator’s insufficient skills (chance node).
The newborns detected as positive (whether true or false) by the clinical Auditory Brainstem Response (ABR) device-as the gold standard-are considered to be definitely diagnosed. An audiologist performs this test, and the model presumes that its accuracy is 100%. The remaining newborns, whose results are negative (whether true or false) are discharged and not followed up (terminal node).
Each device has four branches and end nodes, and their expected cost is determined as follows:
- Branch A/A’ : The cost of screening and definite diagnosis of newborns, reflecting with true positive hearing loss, is included under this branch.
- Branch B/B’ : The cost of screening for newborns, showing false negative hearing loss, is included under this branch.
- Branch C/C’ : The cost of screening and definite diagnosis of newborns, showing false positive normal hearing, is included under this branch.
- Branch D/D’ : The cost of screening for newborns, showing true negative normal hearing, is included under this branch.
The total costs of these four branches indicate the total cost of each device in NHS. Our expected effectiveness for each device was calculated by multiplying the number of newborns entering the model by prevalence, and by device sensitivity.
3.2. Model Inputs
The main inputs of this model include the prevalence of hearing loss in Iran, device sensitivity and specificity, the cost of screening, and definite diagnosis of each newborn. Upon collection, these inputs were analyzed with TreeAge economic analysis software.
The data related to device sensitivity and specificity were collected through a recent systematic review and meta-analysis. This study was based on Cochrane Institute’s standard method for diagnostic accuracy studies.
Only one research has been conducted to analyze the sensitivity and specificity of the OAE, which was meta-analyzed in a systematic review by Heidari et al. [18]. No study was found investigating the sensitivity and specificity of AABR devices. Given that sensitivity and specificity are among the technical specifications of the devices and they are not affected by geographical and local environmental factors, it seems that meta-analysis studies conducted in other countries can be generalized to similar studies in Iran.
Furthermore, to extract relevant data on the prevalence of hearing loss, we focused mainly on the high quality, up-to-date studies with large sample sizes conducted in Iran. Thus, we searched the most important domestic databases such as Magiran, SID, and IranMedex, using the following keywords: ‘Hearing loss’, ‘newborn’ and ‘prevalence’.
The economic analysis in this study was conducted from the perspective of the healthcare system on evaluating cost-effectiveness. In this study, the cost of the newborns’ screening and the cost of definite diagnosis of newborns’ hearing ability were calculated based on the sources of cost used in hearing screening and definite diagnosis, and not based on the costs in private clinics.
To determine the costs, the sources of costs were identified first, and then the amount of each source was quantified and evaluated. Only the direct costs were considered to identify the sources. The unit cost was determined in two steps: In the first step, the unit cost of each of the devices was outlined for screening; and in the second step, the unit cost of the gold standard was outlined. In these two steps, cost findings include the costs of device purchase, repair and maintenance, annual depreciation, location, consumer products, required infrastructures, employees’ salaries and wages, human resources training, overhead costs, taxes and other direct costs. Based on these costs and the variables presented in Table 1, the unit cost per newborn was estimated.
| Parameter | Baseline (Range), $ | Reference | ||
|---|---|---|---|---|
| AABR | OAE | Gold standard | ||
| Device purchase | 5,503 - 7,153 | 4,127 - 5,777 | 17,882 - 22,009 | AE |
| Repair and maintenance during a year | 165 - 220 | 110 - 165 | 413 - 551 | AE |
| Infrastructure | 0 | 0 | 2,751 - 4,127 | AE |
| Testing and general supplies cost per newborn | 0.58 - 0.72 | 0.33 - 0.47 | 1.1 - 1.38 | AE |
| The monthly salary of a human resource | 771 - 881 | 771 - 881 | 771 - 881 | AE |
| Location (monthly rent) | 0 | 0 | 193 - 358 | AE |
| Monthly overhead | 0 | 0 | 110 - 165 | AE |
Abbreviation: AE, authors estimate.
Through contacting five audiology equipment manufacturers either by phone or in person posing as a customer, we obtained information about each device’s cost, lifespan, and salvage value across the country. The remaining sources of cost and the variables presented in Table 2 were designed in the form of a questionnaire. Fifteen experienced audiologists employed in centers offering active UNHS programs completed the questionnaire. Eventually, after collecting the questionnaires, Delphi method was applied to analyze and summarize them.
| Variables | AABR | OAE | Gold Standard |
|---|---|---|---|
| The device’s lifespan | 6 years | 6 years | 6 years |
| Salvage value | $0 | $0 | $0 |
| Average duration of test for one newborn | 17 min | 12 min | 60 min |
| Average duration of device function in one day | 3 hour | 2 hour | |
| Mean screening of newborns in one day | 11 infants | 15 infants | 2 infants |
| Average number of working days in a year | 288 days | ||
| Mean screening of newborns in one year | 3,168 infants | 4,320 infants | 576 infants |
Since the costs were calculated based on the currency in Iran, the exchange rate of 36,350 Iranian Rial (IRR) was used to convert the costs into the U.S. dollar.
In this study, attempt was made to examine the direct costs of human resources. These costs covered the salary and benefits of an audiologist and/or a technician or a secretary, and they did not require training. Moreover, location, overhead and infrastructure costs were not taken into account, because the devices are now portable and the screening test can be performed at the mother’s bedside or in the newborn’s special bed during the first 24 hours of life before the mother is discharged from the hospital.
Since there is no manufacturing company in Iran that recycles scrapped devices, zero was assigned to the salvage value of the devices.
Finally, upon examining the probability of uncertainty concerning the inputs, particularly cost data and the prevalence rate of hearing loss, sensitivity analysis was conducted in view of the maximum and minimum values of these parameters (with the assumption of keeping the other parameters constant).
4. Results
4.1. Sensitivity and Specificity
Based on the systematic review and meta-analysis conducted by Heidari et al. (18) on the sensitivity and specificity of AABR and OAE devices compared to the ABR device (as the gold standard), the pooled sensitivity and specificity of the AABR device were reported to be 0.93 and 0.97, respectively. These figures were 0.77 and 0.93 for the OAE device, respectively (Figure 1).
4.2. The Prevalence of Hearing Loss and the Annual Birth Rates in Iran
Based on the study conducted by Firoozbakht et al. (2), the prevalence of congenital hearing loss in Iran varies from two to eight in 1,000 live births, which has been estimated to be five in 1,000 live births on average. The annual birth rate in Iran has been estimated to be one million on average (19). In general, between 2,000 and 8,000 (a mean of 5,000) newborns are born with permanent congenital hearing loss in Iran annually.
4.3. Costs
Neonatal screening with the OAE device costs between $1.6 and $2.2. This figure is between $2.3 and $2.9 for the AABR device. On the other hand, the definite diagnosis of a newborn, using the ABR device costs between $19.2 and $22. Mainly, the average cost per newborn screening, using the OAE and AABR devices, was estimated at $1.9 and $2.6, respectively, and it was estimated at $20.6 for the definite diagnosis.
4.4. Cost-Effectiveness Analysis
According to the decision tree and the data presented in Table 3, if hearing screening is performed in a one-million cohort population of newborns (considering the annual birth rate), using the OAE device, it will entail the following probable costs and outcomes:
1) 925,350 newborns with normal hearing will be detected correctly with a cost of $1,758,165.
2) 69,650 healthy newborns will be falsely detected as having hearing loss. With respect to the cost of screening and the cost of the gold standard for 69,650 newborns, it will cost $1,567,125.
3) 3,850 newborns with hearing loss will be correctly detected; and taking into account the cost of the gold standard for this number, it will cost $86,625.
4) 1,150 newborns with hearing loss will be falsely detected as healthy. In addition to a cost of $2,185, they will eventually enter a delayed stage of intervention, followed by its subsequent complications.
If universal hearing screening is carried out with the AABR device in the same population, it will entail the following costs and outcomes:
1) 965,150 newborns with normal hearing will be detected correctly, with the cost of $2,509,390.
2) 29,850 healthy newborns will be falsely detected as having hearing loss. With regards to the cost of screening and the cost of the gold standard, it will cost $692,520.
3) 4,650 newborns with hearing loss will be correctly detected; taking into account the cost of the gold standard for this number, it will cost $107,880.
4) 350 newborns with hearing loss will be falsely detected as healthy. In addition to a cost of $910, they will eventually enter a delayed stage of intervention, followed by its subsequent complications.
The universal NHS entails a cost of $3,310,700, and detects 4,650 newborns with hearing loss for a one-year period and a one-million population of newborns, using the AABR device. If the OAE device is used, the cost will exceed to $3,414, and 3,850 newborns with hearing loss will be diagnosed. Collectively, the AABR device costs $103,400 less than the OAE device, and detects 800 more cases compared to the OAE device. Thus, according to the results, the AABR device imposes fewer costs and has greater effectiveness.
4.5. Sensitivity Analysis
Bearing in mind the minimum prevalence rate, the AABR device is $115,760 less costly than the OAE device, and detects 320 more affected newborns compared with the OAE device. If the maximum prevalence rate is taken into account in the model, the AABR, compared with the OAE device, costs $91,040 less and detects 1,280 more affected newborns.
Upon considering the minimum and maximum costs related to the gold standard, the difference between the cost of the two devices is $48,800 and $158,000 in favor of the AABR device. Under similar circumstances, the AABR can detect 800 more newborns with hearing loss compared to the OAE device.
Considering the minimum cost of screening with the OAE device or the maximum screening cost with the AABR device, the difference between the cost of the two devices in screening favors the OAE device, with $196,600. Nevertheless, it can detect 800 fewer newborns with hearing loss compared to the AABR device.
As there was no vagueness surrounding the diagnostic accuracy of the results of the devices, this parameter did not undergo sensitivity analysis.
5. Discussion
Based on the findings, the unit cost of screening per newborn of the AABR was higher compared to the OAE device. Moreover, if NHS is performed among the live population of newborns over a year, the prevalence of hearing loss will decline in Iran. Therefore, in addition to the high diagnostic accuracy of AABR compared to OAE, and the fact that it entails less costs, the AABR device may prevent delayed interventions in 800 newborns and the subsequent complications that may ensue. The number of false positive results (i.e., the newborns who were healthy but falsely detected as cases) was far less in the AABR method than in the OAE method, imposing less costs (direct, indirect and intangible), stress and anxiety on the newborns’ families.
In this study, effectiveness was defined as the percentage of newborns, whose hearing status was correctly detected by each of the two devices. This effectiveness was grounded on the diagnostic accuracy of the devices. In other countries, three studies have been conducted on the economic analysis of these devices for screening. Although they have defined effectiveness by the number of referred cases, their conclusions are in line with those obtained in this research (20-22).
According to Lin et al. [20], in addition to the lower direct medical and intangible costs of the AABR compared to the OAE device, the number of referred false positives was also significantly smaller. Vohr et al. (21) stated that although the unit cost of newborn screening is slightly higher in the AABR technique than in the OAE technique, its referred cases are fewer. Likewise, Lemons et al. (22) believe that the AABR is a good substitute for the OAE, as it entails fewer referred cases and lower total costs per screened newborn.
Sensitivity analysis results showed that the minimum or maximum prevalence rate of hearing loss had no effects on displacing the dominant technology, and that the AABR device was associated with lower costs and greater effectiveness. The minimum and maximum costs of the gold standard also indicated that the AABR costs less and has greater effectiveness. Upon considering the minimum costs of the OAE or the maximum costs of the AABR, the screening procedure employing the AABR is associated with higher costs and effectiveness. Under such circumstances, determining the cost-effective device depends on the threshold that specifies how much the detection of a newborn with hearing loss before the age of three months is valued in a country.
In this model, it was estimated that if the UNHS program was conducted with the AABR device for a year, the health system would undergo a cost of approximately $3,310,700. However, the health system may have to undergo far more costs to efficiently cover this program as many issues such as equity, access to health services, and the limitations of this study remain to be solved.
5.1. Study Limitations
This analysis was based on a one-million-cohort population of annual births, which overlooked the loss to follow up. Here, it was assumed that the newborns were screened only once during their first 24 hours of birth, and the clinical ABR device only confirmed the rejected cases (positive cases). However, in reality, newborns may be screened many times before a definite diagnosis is reached; and usually, such a diagnosis is achieved through multiple tests. In this study, the focus was chiefly on the direct costs, and the indirect service-related costs were not considered. Hence, the estimate presented in this study for the unit cost may not be representative of the real costs. Here, the numbers of correctly detected cases have been employed as criteria for effectiveness, and the outcomes following the definite diagnosis and undetected cases have not been investigated. Such outcomes may include the final effect of hearing loss on language and speech development, communication skills, emotional developments and academic advances. The outcomes of the two groups should have been outlined by considering a wider time horizon, and then, a more realistic CEA of the hearing screening should have been undertaken. In this model, we presumed that an audiologist with the necessary skills performed the screening. Thus, we managed common errors that might have occurred due to the operator’s insufficient skills. In reality, insufficiently skilled operators may perform the screening, as there may be a lack of skilled audiologists. Thence, the error created by the operator can affect the screening results.
We recommend conducting further studies in which costs are considered from the public’s viewpoint, with a wider time horizon, and employing quality of life as a measure for effectiveness. Furthermore, we recommend designing a model for neonatal screening that reflects the operator’s error, loss to follow-up, and the other aforementioned limitations.
5.2. Conclusions
The AABR device is a non-invasive, rapid, safe and simple technology that can be employed in UNHS programs. In case of shortage of skilled and expert work force, it can be easily taught to other personnel. The high sensitivity and specificity of this device, compared to that of the OAE device, not only reduces the number of falsely referred cases, but also detects a greater percentage of newborns with hearing loss. Eventually, better clinical effectiveness may be achieved. Furthermore, considering the annual birth rate, the prevalence rate of hearing loss, and the high diagnostic accuracy of this device in the long run, it can be stated that this device imposes lower costs than the OAE device. In conclusion, if the required infrastructure is provided for UNHS programs, the aforementioned technology can be used as a cost-effective tool in such programs.