1. Background
One of the most common problems noted in newborn hearing screening (NHS) programmes is the high rate of infants that are lost to follow-up at various stages of the programme (1, 2). With the development and progression of NHS, particularly universal newborn hearing screening (UNHS), one of the areas of focus has been the challenge of reducing factors contributing to poor follow-up return rate following referral from NHS (3).
Loss to follow-up within early hearing detection and intervention (EHDI) programmes has both clinical as well as epidemiological implications. Clinically, poor follow-up undermines the effectiveness of early detection of hearing loss and subsequently, provision of early intervention (4, 5). Epidemiologically, it leads to inaccurate data regarding prevalence and incidence of hearing impairment (2, 4); which has significant implications for health systems planning and setting of health priorities for any given context.
Developed countries with successful UNHS programmes suggest that challenges related to follow-up can be lessened by addressing socio-demographic and economic factors (6). The American Speech-Language Hearing Association (ASHA) has considered system issues such as follow-up by primary care providers, lack of communication and coordination among health care providers; as well as access to funding for EHDI to have influence on follow-up (7). Family issues related to caregiver level of education and infant factors have also been considered as contributing to loss to follow-up (7). Provision of appropriate information to families as a means of contributing to high-quality follow-up has also been explored (8). Recommendations regarding provision of information that entails details about what the EHDI process involves as well as reasons for repeated measures and waiting periods between screening or assessment sessions have been made. Furthermore, sharing of appropriate information regarding easy access to professionals to obtain unbiased information that facilitates decision making has also been highlighted (8). Whilst these are some of the services that may assist in ensuring follow-up, professionals also need to consider contextual reasons for poor follow-up return rate, particularly in developing countries where facilities for the effective tracking of mothers are lacking (3, 9).
South Africa does not have a national database for the effective tracking of newborns and infants enrolled in NHS programmes. This lack of a tracking system co-occurs with the lack of prioritisation of data management (10) and a paucity of research related to reasons associated with follow-up default. It is therefore important to investigate follow-up return rate and reasons affecting follow-up as it is one of the key indicators of successful NHS programmes. This is why the current study aimed to explore the factors associated with follow-up return rate within a risk-based NHS programme within the South African context.
2. Methods
2.1. Research Design
A descriptive, longitudinal, repeated measure, within-subjects design was employed (11).
2.2. Participants
The sample consisted of 325 high-risk neonates who were discharged from neonatal intensive care unit (NICU) and high care wards to “step down” wards. Participants were regarded as high-risk due to their presenting medical conditions, and admission to NICU and/or high care wards.
Two hundred and twenty four (69%) participants were delivered by caesarean section and 101 (31.1%) by natural vaginal delivery. The median birth weight was 1390 grams (interquartile range-IQR 1190 - 1555 grams; range 690 - 4020 grams). Mean gestational age was 31.3 weeks (SD 2.8 weeks; range 25-41 weeks) (12).
During hospital stay, 114 (35.1%) participants received ventilation via continuous positive airway pressure and/or intermittent positive pressure ventilation.Two hundred and seventy participants were diagnosed with either neonatal jaundice, hyperbilirubinemia or kernicterus, of which, 243 (90.0%) received phototherapy, seven (2.6%) received phototherapy and exchange blood transfusion, 10 (3.7%) received no treatment, and the treatment of 10 (3.7%) participants was not documented in files.
In-utero infections were present in five (1.5%) participants. Two presented with congenital syphilis, one had congenital rubella, one had congenital cytomegalovirus and one presented with X-ray features of congenital rubella. With regard to syndromes and neurological diagnoses, only five (1.5%) presented with a syndrome or dysmorphic features associated with a syndrome, while 29 participants presented with some form of neurological condition.
2.3. Procedures
This study was conducted at two public sector, academic hospitals in Gauteng, South Africa. Audiological screening and diagnostic procedures were performed by the same audiologist. Participants were recruited over a one year, 11 month period. Neonates admitted to the NICU or high care wards (after birth) and transferred to “step down” wards, for which caregiver consent was obtained, were included in this study. Neonates who were previously discharged, returned home and were readmitted, were not enrolled in this study.
All participants were screened in hospital using transient evoked otoacoustic emissions (TEOAE), distortion product otoacoustic emissions (DPOAE) and automated auditory brainstem response (AABR). All participants were booked for a repeat screening at the hospital, on the same day as their first neonatal follow-up visit (which is six weeks post-discharge). Participants who passed the repeat screening (those who obtained a pass result on all three screening measures bilaterally) were booked for behavioural audiological assessment at the university clinic at six months corrected age for purposes of risk-based surveillance conducted in order to identify late onset hearing loss. Participants who referred on repeat screening (those who obtained a refer result on any one or more of the screening measures in one or both ears) were booked for diagnostic auditory brainstem response (ABR) testing at the university clinic. Participants that passed the AABR at the repeat screening but referred on TEOAE and DPOAE underwent high frequency tympanometry and were booked for a rescreen three weeks later (12). This study protocol differs from standard NHS protocols already established in this geographic region and from recommended protocols in general. Most clinical screening programmes include one or two measures, e.g. DPOAE or TEOAE and/or AABR whereas this study employed all three measures within a repeated measures design. For example, in one study (13), all neonates were evaluated by TEOAE initially (10 to 15 days after birth), with repeat screening if results were refer and ABR at 3 months. The current study’s protocol was unique to the study as both the public sector hospitals did not have fully implemented NHS services at the time of the study.
Adequate follow up return rate was facilitated by telephonic reminders. Reasons for non-attendance for follow up were recorded when contacting caregivers telephonically. Caregivers were provided with funding toward transport costs to facilitate follow-up. Funding was only provided for diagnostic evaluation as the initial and repeat screenings were at the hospital.
2.4. Data Analysis
Descriptive statistics were used to describe factors associated with follow-up return rate. Association between maternal case history factors and whether or not participants returned for follow-up assessments was determined by the chi-squared test for categorical variables and the independent samples t-test for continuous variables. Fisher’s exact test was used for 2 × 2 contingency tables or where requirements for the chi-squared test could not be met. Where the data did not meet the assumptions of these t-tests, a non-parametric alternative, the Wilcoxon rank sum test was used (14).
2.5. Compliance with Ethical Standards
Prior to the study being conducted, ethical clearance was obtained from the University’s Medical ethics committee and permission was also obtained from relevant authorities at the research sites. Written informed consent was obtained from the caregivers of participants. The world medical association declaration of Helsinki’s statement of ethical principles was adhered to during the study where ethical principles of confidentiality, anonymity as well as beneficence and non-maleficence were observed (15).
3. Results
Analysis of the overall outcome of all three measures combined, indicated that more than half of the sample (59.1%) passed the initial screening, compared to those who referred (40.9%). Despite the initial screening outcome, all participants were booked for a repeat screening (six weeks post discharge) on the same day as their first neonatal follow-up visit at the hospital.
Follow-up return rate and factors associated with the follow-up return rate were determined for the repeat screening and diagnostic audiological assessments. The percentage of participants that returned for follow-up are presented in Table 1.
| Session | Expected Participants, No. | Participants That Attended, No. | Participants Lost to Follow-Up, % |
|---|---|---|---|
| Repeat hearing screening | 325 | 216 | 33.5 |
| Rescreen | 24 | 10 | 58.3 |
| Diagnostic assessment | 202 | 93 | 53.9 |
3.1. Follow-Up at Repeat Hearing Screening
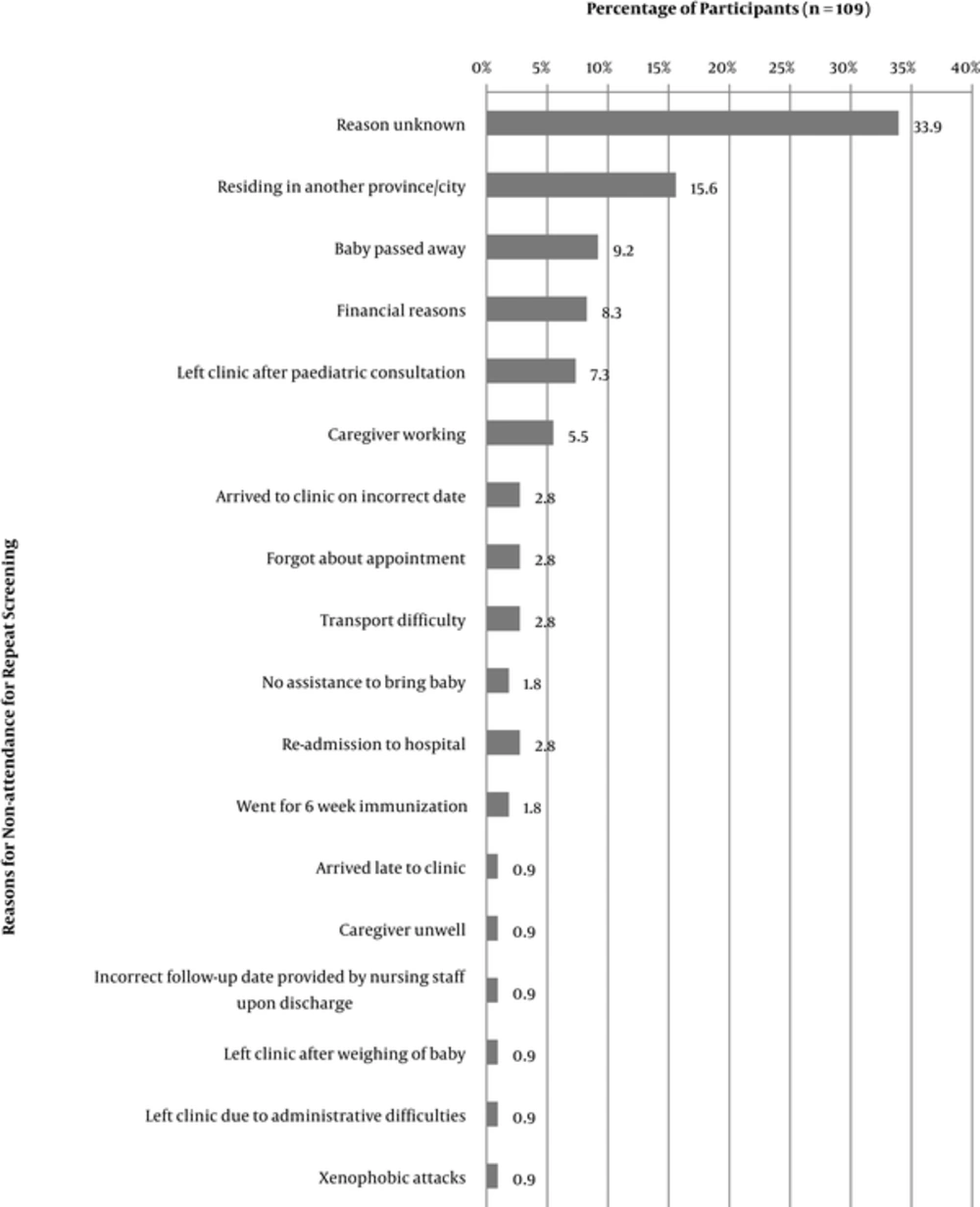
Of the 325 participants booked for a repeat screening, 216 (66.5%) returned; and 109 did not return. Reasons for non-attendance could not be established for 33.9% of the sub-sample as caregivers could not be reached telephonically. For the remaining participants; reasons varied; with the most common reasons for non-attendance at the repeat screening being change of residential location (15.6%), death of the infant (9.2%); and financial reasons (8.3%) (Figure 1).
Of the 216 participants that underwent a repeat screening, 24 were scheduled for a rescreening three weeks following the repeat screening as a result of an incomplete repeat screening. Ten of these participants attended the rescreening. Of the 14 participants that did not attend, reasons for non-attendance were unknown for 10 participants as caregivers of participants could not be reached telephonically. Three participants did not attend due to the caregiver working on that day, and the caregiver of one participant had forgotten about the appointment.
3.2. Follow-Up at Diagnostic Assessment
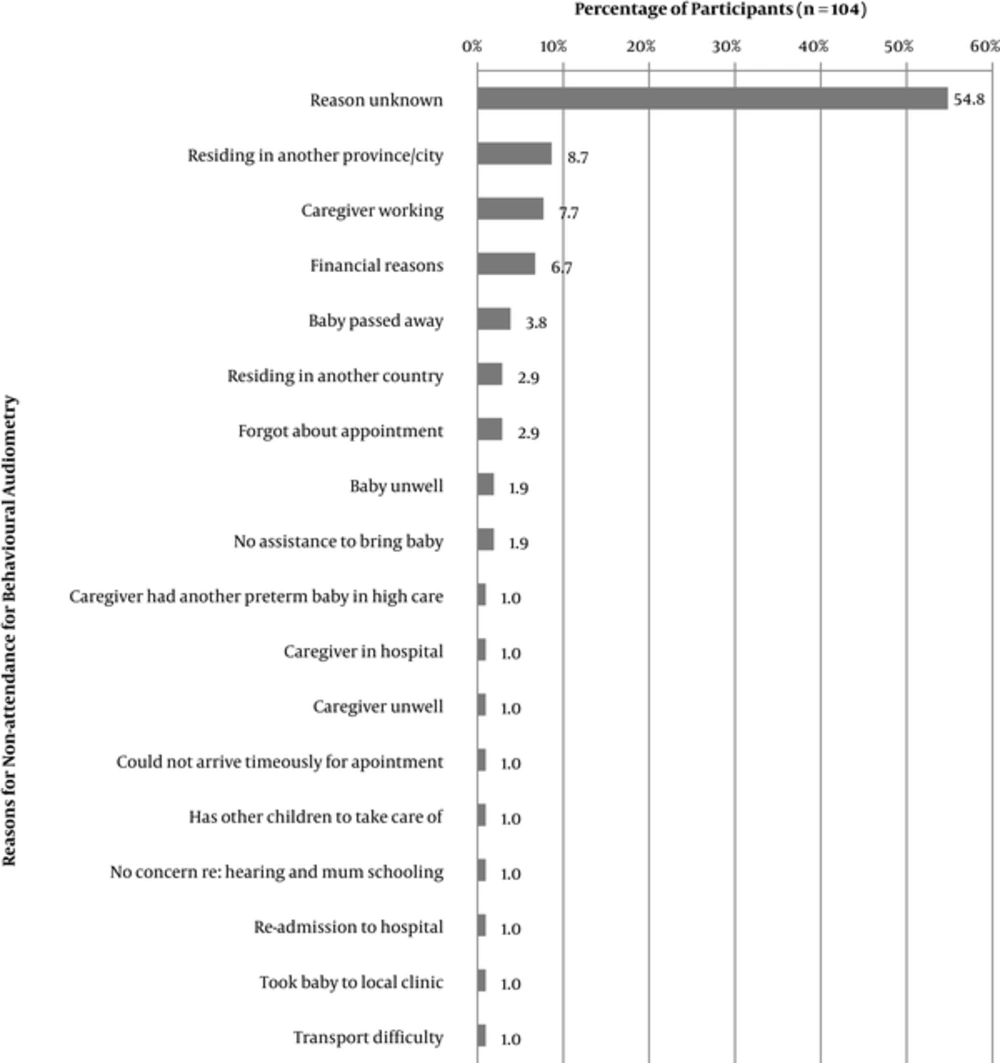
Based on the outcome of the repeat hearing screening, as well as the attendance and outcome at the rescreen, 202 participants were scheduled for diagnostic audiological assessment (191 for behavioural audiometry and 11 for diagnostic auditory brainstem response audiometry-ABR). Of these participants, 93 attended the diagnostic assessment session. Eighty seven of the 93 participants attended a diagnostic behavioural assessment at six months corrected age, while six underwent diagnostic ABR. Follow-up return rate decreased significantly to below 50% for diagnostic assessment. Reasons for non-attendance are illustrated in Figure 2, with over half of the participants having been unreachable telephonically. Reasons for non-attendance varied with the most common reasons being caregivers residing in another province/city (8.7%), caregiver employment (7.7%), and financial reasons (6.7%). Reasons for non-attendance for diagnostic ABR assessment were unknown for three participants, one participant was ill and the other had passed away (Figure 2).
3.3. Association Between Case History Factors and Follow-Up
The association between various case history factors and whether or not participants returned for the repeat screening, and behavioural audiometry at six months corrected age was investigated. These case history factors were based on previous studies that explored follow-up return rate in NHS programmes (16, 17). Analysis of the association between the various factors and follow-up at the repeat screening was conducted using a sample of 315 participants, as 10 infants had passed away and could thus not have attended the screening (Table 2).
| Variable | Overall (N = 315) | Attended Screening | P Valueb | |
|---|---|---|---|---|
| No (N = 99) | Yes (N = 216) | |||
| Hospital | < 0.0001 | |||
| Hospital 1 | 70 (22.2) | 40 (57.1) | 30 (42.9) | |
| Hospital 2 | 245 (77.8 | 59 (24.1) | 186 (75.9) | |
| Maternal education | 1.00 | |||
| Secondary | 265 (84.9) | 82 (30.9) | 183 (69.1) | |
| Tertiary | 47 (15.1) | 14 (29.8) | 33 (70.2) | |
| First child | 0.81 | |||
| No | 186 (59.0) | 57 (30.6) | 129 (69.4) | |
| Yes | 129 (41.0) | 429 (32.6) | 87 (67.4) | |
| Maternal age | 28.1 ± 6.3 | 27.4 ± 6.5 | 28.5 ± 6.2 | 0.14 |
aValues are expressed as No. (%) or mean ± SD.
bP value for test for significant between-group difference.
Follow-up differed between the two hospitals as 42.9% of infants from Hospital 1 returned, while a significantly higher percentage (75.9%) from Hospital 2 returned. Results indicated a significant but weak association between the hospital and whether or not infants returned for the repeat screening (Fisher’s exact test: P < 0.0001; phi coefficient = 0.30).
There was no significant association between maternal age, education level, whether or not this was the first child, and whether or not participants returned for the repeat screening (Fisher’s exact test: P = 0.81).
Analysis of the association between the various factors and follow-up at the behavioural assessment was conducted using a sample of 187 participants (Table 3). These participants are inclusive of those who did and did not attend the behavioural assessment at six months corrected age. Four participants passed away in the interim and were excluded from analysis.
| Variable | Overall (N = 187) | Attended Screening | P Valueb | |
|---|---|---|---|---|
| No (N = 100) | Yes (N = 87) | |||
| Hospital | 0.21 | |||
| Hospital 1 | 24 (12.8) | 10 (41.7) | 14 (58.3) | |
| Hospital 2 | 163 (87.2) | 90 (55.2) | 73 (44.8) | |
| Maternal education | > 0.99 | |||
| Secondary | 159 (85.0) | 85 (53.5) | 74 (46.5) | |
| Tertiary | 28 (15.0) | 15 (53.6) | 13 (46.4) | |
| First child | 0.76 | |||
| No | 114 (61.0) | 62 (54.4) | 52 (45.6) | |
| Yes | 73 (39.0) | 38 (52.1) | 35 (47.9) | |
| Maternal age | 28.2 ± 6.3 | 27.6 ± 6.1 | 29.7 ± 6.2 | 0.023 |
aValues are expressed as No. (%) or mean ± SD.
bP value for test for significant between-group difference.
Results indicated no significant association between whether or not infants returned for the six month behavioural assessment and the hospital, maternal education level, or first child/not. However, the mean maternal age for those who returned (29.7 years; SD = 6.2 years) was significantly higher than those who did not return (27.6 years; SD = 6.1 years) (P = 0.022). The effect size was small (d = 0.34).
4. Discussion
Current findings indicate that the number of participants declined throughout the course of this study, with the highest attrition rate at the diagnostic follow-up session. Follow-up return rate was better at the repeat screening (66.5%) than for diagnostic assessment (46%). These "lost" newborns may be at higher risk for hearing impairment than the general population, particularly if they have referred on the hearing screening (18). Findings are consistent with results reported from a study on frequency of hearing impairment among full-term newborns in Yazd, Iran; where some newborns did not continue with the follow-up visits (19). They are also in agreement with findings from NHS programmes in maternity hospitals in Brazil (20), where loss to follow-up was lower for diagnostic assessment and ranged from 5% to 66% in public hospitals. One of the proposed reasons in this study was that diagnostic evaluations were conducted outside the maternity hospital (20), as in the current study where the protocol entailed diagnostic follow-up at the University clinic which is away from the hospitals where screening occurred. Contrary to these findings, results from a larger NHS follow-up study in a developed context indicated a 91% return rate (21). These authors concluded that a high follow-up return rate does not necessarily ensure timely intervention; as late diagnosis, conductive hearing impairment and coverage by medical aid are often predictors of late fitting and/or loss to follow-up (21).
Return rates for the repeat screening in the current study differed between the two hospitals where the screening programme was implemented. A better follow-up return rate was noted at hospital 2 (75.9%), where the hospital is specifically a maternal and child health care hospital, as opposed to hospital 1 which is a general hospital. This finding is in alignment with the recommended Health Professions Council of South Africa (2007) benchmark of 70% return rate. These differences between the two hospitals raises the question of the possible influence of factors such as quality of care, antenatal clinic attendance or more specialised medical care at maternal and child health care facilities; all of which require further investigation. High follow-up return rates have also been reported in other maternal and child health care settings in South Africa (22).
For increased reach and higher return rate, Ng et al. (23) report the medical assessment clinic to be an ideal time for hearing screening. These authors suggest that it is less costly for parents to attend hearing screening at the medical assessment clinic as parents are able to see several professionals at one appointment instead of attending several clinics for various appointments. This recommendation was tested in the current study, where the repeat screening was aligned with neonatal follow-up at the medical assessment clinic - which involves medical evaluation by paediatricians. Current findings support this healthcare model of aligning audiological and medical appointments, particularly for high risk newborns who undergo medical follow-up in the hospital setting; especially in resource constrained contexts where financial resources influence health seeking behaviours. Current authors believe that this model would increase the numbers of babies screened, and significantly improve the return rate for screening prior to referral to established diagnostic audiology assessment clinics which would offer habilitation services once diagnosis of hearing impairment is made.
Findings from the current study are further supported by earlier suggestions in the literature which advocate for integration of NHS into other federal growth and development monitoring programmes (24). Furthermore, coinciding hearing screening with immunization visits has also previously been proposed in South Africa and Northwest India (25, 26).
Current findings on the overall follow-up return rate are also in accordance with reports from earlier published literature on hearing screening in NICU (27). For example, Lieu and colleagues (27) and the ASHA (7) working group suggest that lack of follow-up may be due to inadequate resources to conduct timely diagnostic ABR assessment; parents and/or paediatricians disregarding the scheduling of diagnostic ABR testing; a newborn population with other medical priorities or needs; lack of parental reminders about follow-up testing; or socioeconomic factors (27).
Although reasons for non-attendance were unknown for a large proportion of participants in the current study due to inability to reach participants telephonically; migration to another province or city; caregiver employment; and financial difficulties such as lack of funds for transport were among the more commonly established reasons. Migration to another province or city is reflective of the South African context where socioeconomic factors, including employment opportunities lead to forced migration. Current findings demonstrate that reasons for follow-up are contextual and highlight the need for an effective data management system, as well as well-established referral pathways to ensure follow-up and tracking of newborns enrolled in EHDI programmes regardless of where they are in the country.
Current findings with regards to the association between maternal age, maternal education, presence of other children and whether or not caregivers returned for the repeat screening revealed that there was no statistically significant association. However, the mean maternal age for those who returned for diagnostic assessment was significantly higher than the mean maternal age of those who did not return. Cavalcanti and colleagues (16) found that mothers with a primary education only, mothers with five or less prenatal visits, and families with a minimum salary or less were more at risk of not attending the second-stage screening. These authors argue that scheduling of appointments may be more difficult in families with more than one child and that these mothers may be more independent and experienced in decision making and less compliant to recommendations or health instructions.
Strategies aimed at facilitating follow-up have been proposed by several authors, some of which were implemented in the current study. These strategies included persistent telephone reminders to caregivers (28), and the use of a second contact name and number (29). Although both these strategies were implemented in the current study, they did not always facilitate follow-up return rate due to various other contextual reasons.
It is the experience of many established screening programmes; in developed countries where follow up services are free at the point of use; that attendance rates in excess of 90% have been achieved for audiological follow up for a screen refer (30). The significant decrease in follow-up return rate for the six month diagnostic assessment in the current study raises questions about the feasibility of targeted/risk-based surveillance which has been recommended for the identification of late onset hearing loss in babies who pass NHS but present with risk factors for hearing loss (31). More recently, the risk-based surveillance model of service delivery has been questioned by some well-established programmes (32). Wood, Davis and Sutton (33) found that uptake for the targeted surveillance appointments was low, consistent with findings from the current study. Wood and colleagues argue that surveillance appointments may not be valued as important by families as they may feel reassured by the NHS result; combined with their own observations of their child’s auditory behaviour and response to stimuli. These authors further argue that the presence of other medical conditions in high-risk infants may result in other, frequent appointments, resulting in hearing not being perceived as priority when compared with those other medical conditions (33).
4.1. Conclusions
Current findings suggest that follow-up return rate remains a significant challenge to NHS and that reasons for non-attendance are contextual and require contextually responsive solutions. However, appropriate alignment of audiological follow-up with other medical follow-up appointments is advantageous. Current findings highlight the importance of taking careful cognizance of the reasons for poor return rate during planning and implementation of EHDI programmes; as well as involving the departments of childcare services and social development for improved follow-up.