1. Background
In 2013, American college of obstetricians and gynecologists (ACOG) suggested neonatal outcomes between 37 - 42 weeks gestation vary depending on the week of delivery and they defined the term pregnancies as ‘late preterm’ (LPT) of gestational age (GA) 340/7 - 366/7 weeks, ‘early-term’ of GA 370/7 - 386/7 weeks and ‘term’ GA of 390/7 - 406/7 weeks (1).
Early-terms account for 27% of all deliveries where infants born at this period encounter increased risk of mortality, neonatal intensive care unit (NICU) admission due to respiratory, neurological and metabolic morbidities (2-6). However, today we know that the clinical prognosis of infants is not as homogenous as expected (1). Classifying the gestations of week 37 and 38 as ‘early-term’ is rather controversial as the rate of complications and morbidity rate rises when GA decreases (7, 8). Current data from Canada’s infant birth-death statistics investigating 4.1 million neonates born after 37 weeks, reports increased mortality of 37 week gestations (4.55/1000 infants at 37 weeks, 2.77/1000 at 38 weeks gestation) (7). In addition, in the recent decades cesarean section (CS) deliveries have an escalating trend at an average of one in four births across organisation for economic co-operation and development (OECD) countries and OECD-2015 data reports Turkey as the leading country with the highest rank of CS deliveries (50.4 CS delivery per 100 live births) (8, 9). Clinicians’ general attitude towards underestimation of negative effects of CS on lung maturity and treating the ‘37 week’ gestations as normal term newborns has led us to conduct a retrospective cohort study on infants born at this gestational age to determine the antenatal characteristics, respiratory morbidity and mortality cases. Our second objective was to compare the differences between 340/7 - 366/7 (LPT infants) with 380/7 - 406/7 gestational weeks (full-term infants), since previous studies mostly focus on either LPT and early-term or early-term and full-term newborns (3, 7, 10, 11).
2. Methods
This retrospective cohort study was conducted in 525 infants who were admitted to NICU of a tertiary neonatal care facility following approval of the ethical institutional board. Eleven patients were excluded due to lack of data or difficulty in diagnosis (pneumonia-diagnosed patients with meconium stained deliveries) and a total of 514 patients were analyzed. Infants encountering clinical signs of respiratory distress within 24 hours of birth between the periods January 2010 - June 2012 were enrolled. Exclusion criteria were defined as infants of GA less than 34 weeks, diagnosis of genetic or metabolic disorders and congenital cardiac anomalies except for patent ductus arteriosus (PDA). In order to compare the differences, infants were categorized into three groups as: LPTs (GA: 340/7 - 366/7 weeks), early-term (GA: 370/7 - 376/7 weeks) and full-term infants (GA 380/7 - 406/7 weeks). Prenatal data included mother’s age, parity, GA (pregnancy duration assessed by last menstrual period or first/ second trimester ultrasound scans), twin birth, antenatal steroid therapy (Celestone Chronodose Injection®: Betamethasone sodium phosphate/betamethasone acetate), presence of oligohydramnios, pre-eclampsia, placenta previa, newborn of gestational diabetic mother (GDM), presence of premature rupture of membranes (PROM), mode of delivery (vaginal birth or CS delivery) and birth place (whether the infant was inborn or outborn - given birth at another facility and later transferred to our unit following development of respiratory distress).
Neonatal demographic data included birth weight, small for gestational age (SGA) or large for gestational age (LGA), sex, 5 minute Apgar score and perinatal asphyxia. Clinical data included signs of respiratory distress and if received the type of assisted ventilation, presence of PDA, pneumothorax and persistent pulmonary hypertension of the newborn (PPHN). Chest X-ray and blood gas analysis, hemogram, C-reactive protein levels were also obtained. Based on the clinical and laboratory features, we classified the respiratory problems as respiratory distress syndrome (RDS), transient tachypnea of newborn (TTN), meconium aspiration syndrome (MAS) and congenital pneumonia. Patients received supplemental O2 by nasal cannula or hood, nasal continuous positive airway pressure (nCPAP), conventional mechanical ventilation (MV) and if necessary high frequency oscillatory ventilation (HFOV). The ones who were diagnosed with MAS or RDS received intratracheal surfactant therapy. Patients were also analyzed for co-morbidities such as sepsis, hypoglycemia, intracranial hemorrhage (ICH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC) (diagnosis based on radiological/ surgical findings), length of NICU stay, length of MV and mortality.
Data were analyzed using SPSS version 21.0 (SPSS Inc. Chicago, IL). Categorical data was assessed using Chi-square test or Fisher-Exact test. Differences between groups were evaluated by Kruskal-Wallis test; in presence of statistical significance, the post-hoc Bonferroni-Dunn test was applied. For continuous variables, normal distribution was investigated by Shapiro-Wilks test (if group sample ≤ 50) or Kolmogorov-Smirnov test (if group sample > 50). P value of < 0.05 indicated significance.
3. Results
We have enrolled 514 infants suffering from respiratory distress within the 24 hours of birth. Table 1 represents the demographics and the maternal conditions of the study population. CS was the common choice of delivery (71.4%) and the male/female ratio was 1.58. The majority of the infants (332/514, 64.6%) were outborn (born at another facility and later transferred to our NICU after progression of respiratory distress). TTN was the major respiratory disease in all gestational groups (84.4%); followed by RDS (7.8%), MAS (5.8%) and congenital pneumonia (1.9%). Table 2 represents the respiratory problems and morbidities of the study groups. Necessity of assisted ventilation included conventional MV in 131 (25.5%), HFOV in 10 (1.9%) and nCPAP in 81 (15.8%) infants; the rest received supplemental oxygen by hood or nasal cannula. In order to define characteristics and the associated respiratory problems, we categorized patients relying on gestational weeks.
| Variables | No. (%) |
|---|---|
| Demographics | |
| Gestational age, weeks | |
| 340/7 - 366/7 | 184 (35.8) |
| 370/7 - 376/7 | 84 (16.3) |
| 380/7- 406/7 | 246 (47.9) |
| Birth weight, grams | |
| 1001 - 1499 | 8 (1.6) |
| 1500 - 1999 | 57 (11.1) |
| 2000 - 2499 | 106 (20.6) |
| 2500 - 2999 | 173 (33.7) |
| 3000 - 3999 | 149 (29.0) |
| > 4000 | 21 (4.1) |
| Male, % | 315 (61.3) |
| Weight for gestational age | |
| SGA | 35 (6.8) |
| LGA | 21 (4.1) |
| Twin birth | 61 (11.9) |
| Birth place | |
| Inborn | 182 (35.4) |
| Outborn | 332 (64.6) |
| Mode of delivery | |
| CS delivery | 367 (71.4) |
| Vaginal birth | 147 (28.6) |
| PDA | 23 (4.5) |
| Low Apgar scores, ≤ 7 at 5 min. | 77 (15) |
| Maternal Conditions | |
| Maternal age, mean ± SD | 27.42 ± 3.84 |
| Gravida (median) | 2 (1-6) |
| Antenatal steroids | 31 (6.0) |
| PROM | 9 (1.7) |
| GDM | 60 (11.7) |
| Placental conditions | 18 (3.5) |
| Preeclampsia | 32 (6.2) |
| Oligoamniosis | 34 (6.6) |
Abbreviations: CS Delivery, Cesarean Section Delivery; GDM, Gestational Diabetic Mother; LGA, Large for Gestational Age; PDA, Patent Ductus Arteriosus; PROM, Premature Rupture of Placental Membranes; SGA, Small for Gestational Age.
| Variables | No. (%) |
|---|---|
| Respiratory Disease | |
| TTN | 434 (84.4) |
| RDS | 40 (7.8) |
| MAS | 30 (5.8) |
| Congenital pneumonia | 10 (1.9) |
| Ventilation Support | |
| Oxygen | 222 (56.8) |
| nCPAP | 81 (15.8) |
| Conventional MV | 131 (25.5) |
| HFOV | 10 (1.9) |
| Intratracheal surfactant | 70 (13.6) |
| Morbidity | |
| Pneumothorax | 32 (6.2) |
| Pulmonary hypertension | 22 (4.3) |
| Pulmonary bleeding | 5 (1) |
| PVL | 32 (6.2) |
| BPD | 1 (0.2) |
| Hypoglycemia | 77 (15) |
| Sepsis | 28 (5.4) |
| NEC | 8 (1.6) |
| Length of intubation (median) | 3 (1-93) days |
| Length of NICU stay (median) | 5 (3-93) days |
| Mortality | 23 (4.5) |
Abbreviations: BPD, Broncho Pulmonary Dysplasia; HFOV, High Frequency Oscillatory Ventilation; MAS, Meconium Aspiration Syndrome; MV, Mechanical Ventilation; nCPAP, Nasal Continuous Positive Airway Pressure; NEC, Necrotizing Enterocolitis; NICU, Neonatal Intensive Care Unit; PVL, Periventricular Leukomalacia; RDS, Respiratory Distress Syndrome; TTN, Transient Tachypnea of Newborn.
3.1. Early-Term Infants
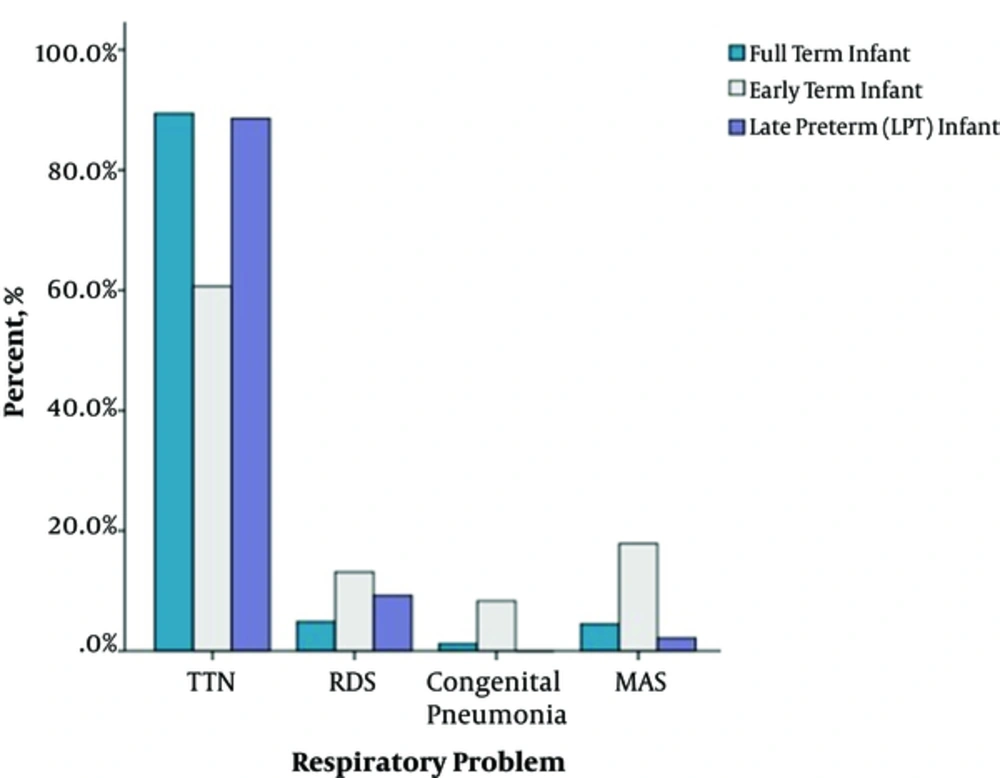
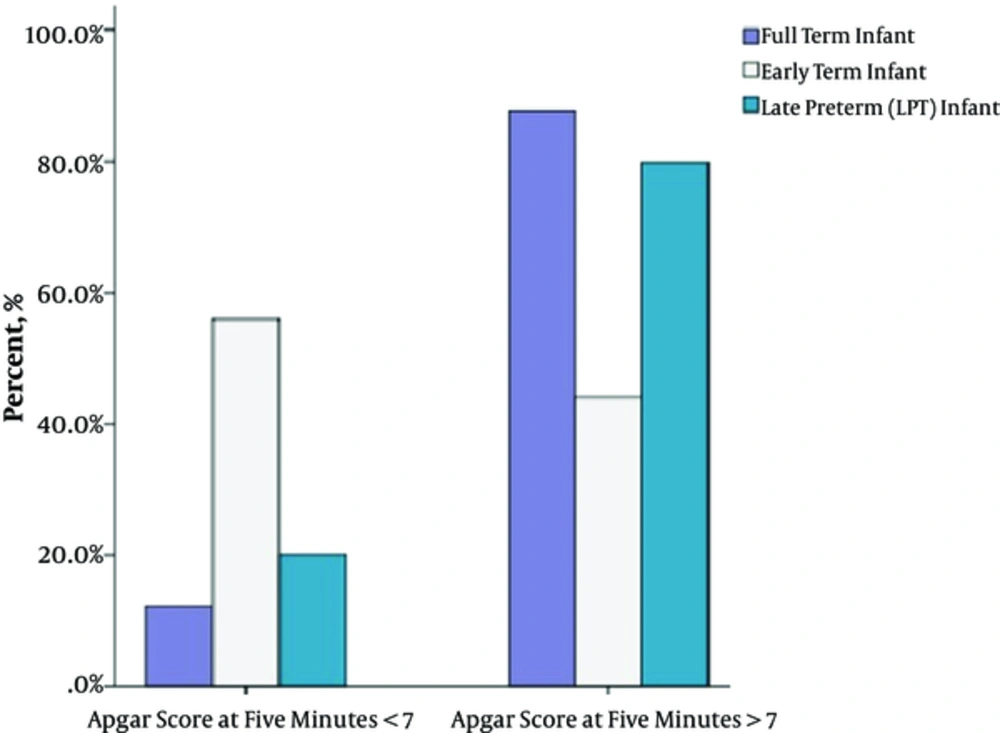
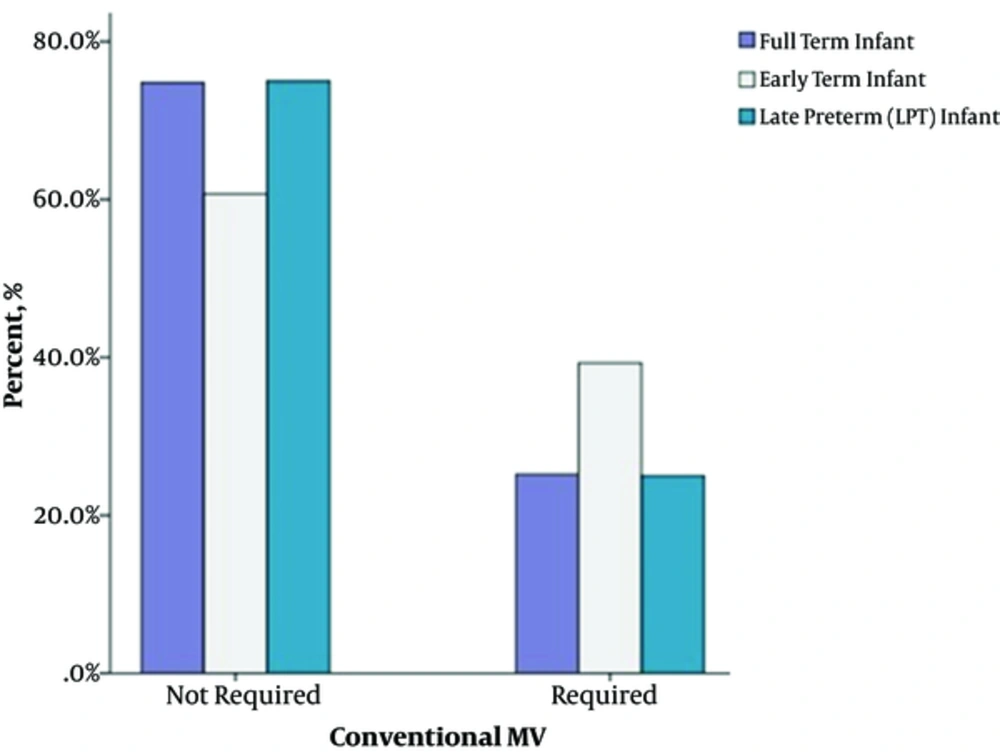
Table 3 shows comparison of the demographics of three gestational groups. Infants of this gestational period were significantly prone to respiratory problems in the present study. Among the whole study group, they had the highest rates of RDS (13.1%; P = 0.033 for early-term and full-term infants; P = 0.338 for early-term and LPT infants) and MAS (P < 0.001 for early-term and LPT infants; P < 0.001 for early-term and full-term infants) (Table 4 and Figure 1). These patients required conventional MV more often compared to LPT and full-term neonates (39.3%; P = 0.050 and P = 0.042 respectively). PVL had a higher occurrence at this gestational group (11.9%, P = 0.033 for early-term and full-term infants; P = 0.567 for early-term and LPT infants). We have also observed lower Apgar scores at 5 minutes (6.1 ± 1.98; P < 0.001 for early-term-full-term infants and P = 0.642 for early-term-LPT infants) and 56% of this gestation demonstrated 5-minute Apgar scores lower than seven (P < 0.001 for both LPT and full-term infants respectively) (Table 3, Figure 2). PPHN (confirmed by echocardiography) was also prominent at this gestation compared to LPTs (P = 0.006), but similar significance was not determined with full-term infants (P = 0.369).
| Variables | LPT, (340/7 - 366/7 Weeks), (N = 184) | Early-Term, (370/7 - 376/7 Weeks), (N = 84) | Full-Term, (GA ≥ 38 Week), (N = 246) | P Value | PL-Ec | PL-Fc | PE-Fc |
|---|---|---|---|---|---|---|---|
| Sex -male | 58.7 (108/184) | 54.8 (46/84) | 65.4 (161/246) | P = 0.148 | - | - | - |
| Twin birth | 26.6 (49/184) | 10.7 (9/84) | 1.2 (3/246) | P < 0.001 | P = 0.009 | P < 0.001 | P < 0.001 |
| CS delivery | 82.1 (151/184) | 61.9 (52/84) | 66.7 (164/246) | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.999 |
| Antenatal Steroids | 16.8 (31/184) | 0 | 0 | NA | - | - | - |
| 5 min Apgar score, mean ± SD | 6.5 ± 1.55 | 6.1 ± 1.98 | 7.93 ± 1.7 | P < 0.001 | P = 0.642 | P < 0.001 | P < 0.001 |
| 5 min Apgar score < 7 | 20.1 (37/184) | 56 (47/84) | 12.2 (30/246) | P < 0.001 | P < 0.001 | P = 0.075 | P < 0.001 |
| Asphyxia | 14.1 (26/184) | 25 (21/84) | 12.2 (30/246) | P = 0.016 | P = 0.090 | P = 0.999 | P = 0.015 |
| Place of Birth (Inborn) | 50.5 (93/184) | 23.8 (20/84) | 28 (69/246) | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.999 |
| Mother Age, mean ± SD | 26.89 ± 1.43 | 26.73 ± 1.81 | 28.04 ± 5.23 | P = 0.002 | P = 0.871+ | P = 0.003+ | P = 0.002+ |
| Oligoamniosis | 6.5 (12/184) | 9.5 (8/84) | 5.7 (14/246) | P = 0.474 | - | - | - |
| Preeclampsia | 9.2 (17/184) | 9.5 (8/84) | 2.8 (7/246) | P = 0.010 | P = 0.999 | P = 0.012 | P = 0.084 |
| Placenta previa | 1.1 (2/184) | 15.5 (13/84) | 1.2 (3/246) | P < 0.001 | P < 0.001 | P = 0.999 | P < 0.001 |
| Diabetic mother | 9.8 (18/184) | 19 (16/84) | 10.6(26/246) | P = 0.069 | - | - | - |
| PROM | 3.8 (7/184) | 0 | 0.8 (2/246) | NA | - | - | - |
Abbreviations: LPT, Late Preterm Infant; PROM, Premature Rupture of Placental Membranes.
aDirect comparisons between two groups: PL-E: LPT and Early-term groups; PL-F: LPT and Full-term groups; PE-F: Early-term and Full-term groups.
bValues are expressed as number percentage.
cBonferonni adjusted P value; +Tamhane adjusted P value.
| Variables | LPT, (GA 340/7 - 366/7 Weeks), (N = 184) | Early-Term, (GA 370/7 - 376/7 Weeks), (N = 84) | Full Term, (GA ≥ 38 Weeks), (N = 246) | P Value | PL-Ec | PL-Fc | PE-Fc |
|---|---|---|---|---|---|---|---|
| TTN | 88.6 (163/184) | 60.7 (51/84) | 89.4 (220/246) | P < 0.001 | P < 0.001 | P = 0.999 | P < 0.001 |
| RDS | 9.2 (17/184) | 13.1 (11/84) | 4.9 (12/246) | P = 0.034 | P = 0.338 | P = 0.222 | P = 0.033 |
| MAS | 2.2 (4/184) | 17.9 (15/84) | 4.5 (11/246) | P < 0.001 | P < 0.001 | P = 0.597 | P < 0.001 |
| Congenital Pneumonia | 0 | 8.3 (7/84) | 1.2 (3/246) | NA | |||
| Conventional MV | 25.0 (46/184) | 39.3 (33/84) | 25.2 (62/246) | P = 0.029 | P = 0.050 | P = 0.999 | P = 0.042 |
| HFOV | 1.6 (3/184) | 3.6 (3/84) | 1.6 (4/246) | NA | |||
| nCPAP | 13.1 (24/184) | 15.5 (13/84) | 17.9 (44/246) | P = 0.291 | |||
| Length of intubation (median), days | 4 (3 - 67) | 3 (2 - 61) | 2 (1 - 93) | P < 0.001 | P = 0.014 | P < 0.001 | P = 0.030 |
| Pneumothorax | 6 (11/184) | 3.6 (3/84) | 7.3 (18/246) | P = 0.464 | |||
| PPHN | 1.1 (2/184) | 9.5 (8/84) | 4.9 (12/246) | P = 0.005 | P = 0.006 | P = 0.084 | P = 0.369 |
| PDA | 2.2 (4/184) | 8.3 (7/84) | 4.9 (12/246) | P = 0.071 | |||
| NEC | 3.3 (6/184) | 1.2 (1/84) | 0.4 (1/246) | NA | |||
| Co-morbidity and Mortality | |||||||
| LPT, (GA 340/7 - 366/7 Weeks), (N = 184) | Early-Term, (GA 370/7 - 376/7 Weeks), (N = 84) | Full Term, (GA ≥ 38 Weeks), (N = 246) | P Value | PL-Ec | PL-Fc | PE-Fc | |
| Length of NICU stay (median), days | 7 (6 - 67) | 4 (3 - 61) | 3 (3 - 93) | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Deceased | 2.2 (4/184) | 8.3 (7/84) | 4.9 (12/246) | P = 0.071 | |||
| Time of death (median), days | 22 (5 - 67) | 9 (3 - 61) | 13 (3 - 93) | P = 0.833 | |||
| Hypoglycemia | 22.8 (42/184) | 14.3 (12/84) | 9,3 (23/246) | P = 0.001 | P = 0.318 | P < 0.001 | P = 0.615 |
| Sepsis | 9.8 (18/184) | 4.8 (4/84) | 2.4 (6/246) | P = 0.004 | P = 0.504 | P = 0.003 | P = 0.839 |
| Culture positivity in sepsis | 66.7 (12/18) | 50 (2/4) | 16.7 (1/6) | NA | |||
| ICH | 7.1 (13/184) | 11.9 (10/84) | 5.7 (14/246) | P = 0.163 | |||
| PV | 7.1 (13/184) | 11.9 (10/84) | 3.7 (9/246) | P = 0.022 | P = 0.567 | P = 0.339 | P = 0.033 |
| NEC | 3.3 (6/184) | 1.2 (1/84) | 0.4 (1/246) | NA |
Abbreviations: HFOV, High Frequency Oscillatory Ventilation; ICH, intracranial hemorrhage; LPT, Late Preterm Infant; MAS, Meconium Aspiration Syndrome; MV, Mechanical Ventilation; nCPAP, Nasal Continuous Positive Airway Pressure; NEC, Necrotizing Enterocolitis; NICU, neonatal intensive care unit; PDA, Patent Ductus Arteriosus; PPHN, Persistent Pulmonary Hypertension of Newborn; PVL, periventricular leukomalacia; RDS, Respiratory Distress Syndrome; TTN, Transient Tachypnea of Newborn.
aDirect comparisons between two groups: PL-E: LPT and Early-term groups; PL-F: LPT and Full-term groups; PE-F: Early-term and Full term groups.
bValues are expressed as number percentage.
cBonferonni adjusted P value; +Tamhane adjusted P value.
3.2. LPT Infants
Twin births and CS deliveries were observed at significant rates in LPT infants (P < 0.001, P < 0.001). Birth places were marked as mainly inborn and approximately half of the LPT infants were transferred to our unit by intrauterine transports for possible risks of delivering premature babies (Table 3). Moreover, antenatal betamethasone sodium phosphate/betamethasone acetate (Celestone Chronodose Injection®) was administered to 31 (16.8%) women of 340/7 - 366/7 weeks gestation to enhance fetal lung maturity. Frequencies of respiratory problems and the necessity of assisted ventilation in terms of conventional MV, HFOV and nCPAP are described in Table 4. The LPT infants had longer intubation periods (P = 0.014 for LPTs and early-terms; P < 0.001 for LPTs and full-term infants) and NICU stays (P < 0.001 for LPTs and early-term infants; P < 0.001 for LPTs and full-term infants) (Table 4).
Analysis for co-morbidities revealed significant hypoglycemia incidence at this period (P = 0.001) compared to later gestational weeks; however hypoglycemia and being the newborn of GDM were not significant in the study population (P = 0.621). In terms of sepsis, lower gestations (LPT group) were clearly associated with more septic infants (P=0.004) but no infectious etiology could be determined in approximately half of the septic patients (13/28, 46.4%). Methicillin-resistant staphylococcus aureus was the most common detected pathogenic organism (6/15 culture positive infants, 40%). The number of NEC patients was too small to analyze, though the linear by linear association revealed an inversely proportional NEC rate with gestation (P = 0.019).
3.3. Full-Term Infants
At this group the patient demographics revealed older mother age (P = 0.002) and less preeclampsia rates (2.8%, P = 0.010). As expected, they confronted highest TTN rates (89.4%); on the contrary RDS seemed as the least observed respiratory intervention (4.9%). Compared to earlier gestations, they clearly had better outcomes in terms of length of MV and NICU stays (Table 4).
3.4. Mortality
Twenty-three (4.5%) infants died overall. Although mortality rates were not statistically significant between groups (P = 0.071), highest mortality rate was observed at early-term infants (8.3%). Table 4 represents the length of NICU admissions, mortality rates and the associated morbidities.
4. Discussion
Early term (37-week gestation) neonates encounter several respiratory problems and related morbidities. We have observed that these infants presented significant RDS rates similar to 34 - 36 week gestational (late preterm) neonates; on the other hand they seemed to have a tendency to hypoxic deliveries with low Apgar scores. Previous studies regarding early terms mostly consisted of either comparison of early-term and late-preterm (infants born at 340/7 - 366/7weeks) or early-term and full-term infants (infants born after 380/7 week gestation) (3, 7, 10, 11). Thus we decided to analyze our data retrospectively to search the respiratory problems and the co-morbidities of such infants and compared them with both late preterm and full-term infants. Compared to later gestations, increased risks of NICU admission and neonatal respiratory morbidity including TTN, RDS, intubation rates or oxygen demand have been attributed to these infants in previous studies (4, 11-14). They constitute a major health care problem as a result of elevating birth rates at this gestation (3). The present study demonstrated 16.3% of NICU admission rates for early-term infants which were quite similar to previous research reaching up to 17.8% (3, 13).
This period has a tendency to develop RDS compared to later gestations (15-17). Morrison et al. reported the rates of respiratory morbidity by CS-induced iatrogenic prematurity as 73.8/1000 at 37th week of GA, 42.3/1000 at 38th GA and 17.8/1000 at 39th GA which were inversely proportional with gestational weeks (18). Moreover, even if the RDS incidence is lower than in LPT infants, the complications and outcomes can be more severe compared to the former group (3). Increasing trends for CS delivery due to families’ or physicians’ reluctance for vaginal birth also contribute to delays in maturation of neonates’ lungs (3, 19). Considering the incidence of 61.9% CS deliveries at 37 week gestations, it was not surprising for us to observe higher RDS rates compared to later gestations. Supporting the previous literature, we believe the highest RDS rate at early-terms had resulted from lung immaturity due to CS delivery. At this stage we should emphasize that Turkey is the leader in terms of CS ratio according to OECD 2015 data with 50.4 CS deliveries per 100 live births (9). This is shown in Table 1 as 71.4% of all deliveries in our study.
Administration of antenatal betamethasone to 16.8% of 340/7 - 366/7 gestational weeks to prevent RDS could contribute to lesser incidence of LPT infants. Although the effect of antenatal steroids on lung maturity is well established, conflicting recommendations exist regarding the optimal timing of drug administration (19-21). A recent article of ACOG in 2016 suggested the consideration of betamethasone to singleton pregnancies at risk for preterm deliveries between 340/7 - 366/7 weeks (21). This could also be attributed to the paper of Gyamfi-Bannerman et al. with significant outcomes on the topic of antenatal steroids in decreasing the necessity of respiratory support and postnatal resuscitation as well as respiratory complications (20). On the contrary Royal college of obstetricians and gynecologists (RCOG) recommends administering antenatal steroids to planned deliveries less than 38+6 weeks (19). Birth without labor increases the risk of RDS approximately equivalent to the risk of an infant born two weeks earlier (22, 23).
One can already expect MAS occurrence in later gestations (24). Cheng et al. reported lower MAS rates in 37 weeks gestations than 39 weeks gestations with an adjusted ratio of 0.62 (95% CI, 0.52 - 0.74) (25). However our data on MAS incidence was not correlated with previous studies where early-term infants had a ratio of 17.9%, clearly higher than their full-term counterparts (P < 0.001). The inversely proportional MAS ratio with gestation might reflect the intrauterine stress and hypoxia at early-terms presented as lower Apgar scores, higher asphyxia (25%; P = 0.016) and PVL rates (11.9%, P = 0.022) in our study. Parikh et al. demonstrated similar results of high asphyxia incidence and cerebral palsy at 37 week gestations (compared to 38 gestational weeks) (26). The predisposing factor for fetal hypoxia could also be the result of higher placenta previa (15.5%; P < 0.001) or preeclampsia rates (9.5%; P = 0.010) compared to full-term infants. PPHN was also observed more often at 37 gestational weeks which was probably related to the frequency of MAS in this group (9.5%; P = 0.005). Our findings regarding early-terms confirmed the risks of these infants in terms of respiratory interventions compared to later gestations.
The results of this study demonstrated the risks of 37 gestational weeks for respiratory complications. Once the clinical sign of a respiratory distress or necessity of resuscitation occurs, patients are primarily admitted to the newborn nursery and later to the NICU. This might delay the treatment and lead to clinical deterioration if not intervened properly (3). These infants were mostly given birth at another facility and later transferred to our unit with the highest postnatal transfer rates of 76.2%. Higher PVL rates (11.9%, P = 0.022) verifying hypoxia in our study supported the complication rates reported in previous studies (3). Early-terms also necessitated highest rank of conventional MV compared to both LPT and full-term infants (39.3%, P = 0.017).In terms of outcomes, survival rates were not significant between groups unlike the studies demonstrating higher mortality in LPTs (27). Our results were concordant with the study of Teune et al. reporting the need of mechanical ventilation in LPTs as RR, 4.9; 95% CI, 2.8 - 8.6; AR, 2.5% vs. 1.2% compared to neonates of later gestational weeks (28). We have observed increased incidence of hypoglycemia and sepsis in LPT infants as gestational weeks decreased.
There were several new outcomes in this study: one could expect that MAS is observed more frequently in term gestations, on the contrary the ratio was higher in early term gestations in this retrospective analysis. Although previous literature suggests that RDS rate should be prevalent in late preterm infants compared to early terms there was no statistical significance but the numeric values were greater in early terms. A new approach that we derive from this study can be summarized as: infants of late preterm gestations were perceived as having immature lung capacity and even administered surfactant at NICU considering they needed intensive care. On the other hand CS deliveries are dominant choice in Turkey leading to immature respiratory system compared to spontaneous vaginal deliveries and this is often underestimated by clinicians. The obstetricians generally approach 37 week gestations (early terms) as normal term infants having matured lungs not considering the negative effects of CS. Normal vaginal deliveries should be recommended for healthy infants.
There were several limitations regarding our research as retrospective nature of this single-institutional study with relatively small sample size weakened the statistical power. The results were also not sufficient to make any conclusion since this was an institutional-based study rather than a population-based one. We should also emphasize the high CS rates in the study population which makes it impossible to interpret any conclusions on population base. Nevertheless our main objective was to observe the institutional outcomes of 37 gestational week infants and decide whether our results were concordant with previous research. Secondly, we tried to draw attention to inconsistent management of these infants in daily routine at hospital settings; since many of those were approached as full-term neonates. Hence they encounter undeniable morbidity compared to their full-term counterparts, respiratory morbidity might reach to the levels of earlier gestations as well. One other limitation was the quality of data from other facilities as 332 of 514 infants were born in those centers. We have included every single data of each patient in order not to omit anything on their health records.
4.1. Conclusion
In this retrospective study we have tried to elaborate the respiratory morbidities of 37 week gestations that were once regarded as ‘term infants with completed lung maturity’ and also draw attention to the inconsistent routine management of such patients in hospital settings. Early term (37th week gestation) neonates encounter respiratory problems and morbidities and significant RDS rates similar to the rates of 34 - 36 weeks gestational (late preterm) neonates; and seem to have a tendency to hypoxic deliveries with low Apgar scores. Considering the increasing delivery rate at this period, real estimates should be investigated in large, multi-centered prospective studies in terms of both short and long term outcomes. Convincing discussions with the family members should be conducted in order to eliminate elective CS at this gestation and postpone the delivery to later gestational weeks if possible.