1. Background
Cerebral Palsy (CP) is the most common motor disability in childhood (1) and is considered as a collection of motor disorders caused by brain damage before, during or after birth. This can lead to poor coordination, poor balance, abnormal movement patterns or a combination of all these aforementioned characteristics (2). Although the disease itself is not progressive; the appearance of neuropathogenic lesions and their clinical expression can alter during the time and through brain development. The birth prevalence of CP is approximately 2 per 1000 live births (3, 4). CP is etiologically multifactorial. Known causes comprise a small fraction of cases. The most prominent causes are prenatal asphyxia and prematurity (4). CP classification is based on resting tone and involved limbs. Spastic CP is the most common type and includes nearly 80% of all cases. This type is associated with spasticity, hyperreflexia, clonus and upward plantar reflexes. Other types of CP such as dyskinetic or ataxic account for less than 20% of all cases (5). It is diagnosed with a history of developmental motor delay, persistence of postural reflexes and lack of development of protective reflexes especially parachute in the first year of life. CP frequently begins with early hypotonia in first 6 to 12 months of life which then leads to spasticity (6). Spasticity is the most disabling feature of these patients. So attempts to relieve spasticity can dramatically improve patient’s quality of life. Although rehabilitation is the mainstay of therapy, pharmacological interventions may improve the quality of life of these patients. Various medications have been used for motor problems associated with CP. These medications affect spasticity and abnormal movements such as dystonia, myoclonus, chorea and athetosis. Cerebrolysin is a neurotrophic peptide isolated from pig brain. It consists of 75% free amino acids and 25% low molecular weight peptides (< 10 KDA) (7). There are many studies reporting the efficacy of Cerebrolysin on neurologic disorders. However, most of them were performed in Russia and China. The first studies of this drug were developed in 1973. Cerebrolysin was first used in Russia in patients with cerebroarteriosclerosis (8). Moreover, there were some other studies on treating infantile cerebral palsy (9) and geriatric patients (10) with Cerebrolysin but neither control nor the results of these trials were convincing enough. Later trials indicated the positive effects of Cerebrolysin for different neurologic disorders such as improving extrapyramidal hyperkinesis (11, 12) or autism and Asperger’s syndrome (13). Moreover there are so many studies showing the efficacy of Cerebrolysin in treatment of Alzheimer's disease (14-17).
2. Objectives
Only few studies investigated the efficacy of Cerebrolysin on CP. Therefore, we designed a prospective study to evaluate the usefulness of Cerebrolysin in management of spasticity in children with CP.
3. Methods
3.1. Patients
This study is a non-randomized non-controlled clinical trial with registration number of IR.TUMS.REC.1394.142077 and IRCT201107226907N3 which involved a total of 26 patients with CP referred to pediatric neurology clinic of Children’s medical center affiliated to Tehran University of Medical Sciences between July 2013 and June 2014. The inclusion criteria were patients who were diagnosed as CP with spasticity between the ages of 6 and 12 years. Exclusion criteria were patients with active epilepsy or renal failure. 45 CP patients were identified initially. From these study candidates, 19 were excluded because of not matching with inclusion criteria and the remaining outpatients started the process of treatment.
3.2. Modified Ashworth Score
Modified Ashworth score (MAS) is a suitable instrument for the assessment of spasticity which was used in our cases (18). MAS is based on muscle tone; in fact more increase in muscle tone leads to higher score of MAS [Table 1]. All of the measurements in our patients are taken in supine position, head being in midline and the resting limb position was neutral except for hip measurement in external rotation taken in sitting position. The scores for MAS were determined according to the level of resistance during the passive movement of the antagonist muscles. The muscle groups tested were biceps, hip flexors, internal rotators of hip and hamstrings. The sum of all the obtained scores of muscles of each patient yealds a single MAS for each case.
| Score | Modified Ashworth scale |
|---|---|
| 0 | No muscle tone |
| 1 | Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension |
| 1+ (2) | increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM Slight |
| 3 | More marked increase in muscle tone through most of the ROM, but affected part(s) easily moved |
| 4 | Considerable increase in muscle tone, passive movement difficult |
| 5 | Affected part(s) rigid in flexion or extension |
3.3. Methods
All of our subjects were under occupational therapy for more than a year. To realize the role of this therapy in improvement of their current spasticity, 2 months prior to starting the injection of Cerebrolysin, MAS was obtained and then it was assessed again by starting injections. It is notable no changes in the spasticity were observed during these 2 months. Therefore, we could underestimate this confounder (rehabilitation therapy) during our trial.
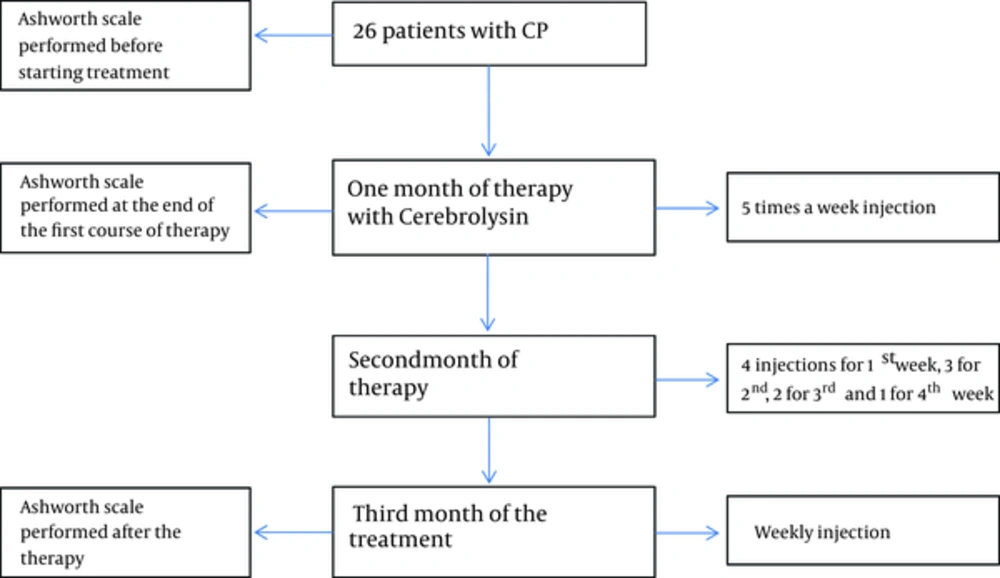
After assessing spasticity, 0.1 cc/kg of Cerebrolysin was administrated intramuscularly into the gluteus muscle. These injections were performed as 5 times per week for the first month. In the second month of therapy, Cerebrolysin injections continued as follows: 4 injections in the first week, 3 injections in the second week, 2 injections in the third week and a single injection in the fourth week. For the third month of therapy, Cerebrolysin was continued as weekly injections (Figure 1). Follow up neurological exams and MAS assesments were done at the end of the first and third month.
3.4. Statistical Analysis
Results of metric variables were presented as means and standard deviations (mean ± SD); also for statistical analysis independent t-test was used. Descriptive variables were described using percentage and to compare Chi-square was used. The obtained data were analyzed by SPSS V.16 software. The statistical significance was set at P value of < 0.05.
4. Results
26 patients diagnosed with Cerebral Palsy were studied in this trial. Among our cases, there were 18 boys and 6 girls (69.2% males, 30.8% females). All clinical characteristics are listed in Table 2. Anatomic, physiologic and functional are the most familiar types of CP classification. Based on each sort, we assessed the types of CP in each one of our outpatients (Table 3). The functional level of participants was classified according to the gross motor function classification system (GMFCS) (19). Level 1 represents the children who can walk and run without restrictions but with decreased speed or balance. Level 2 represents those who can walk with railing and minimal ability to run. Level 3 represents those who can walk with assistive mobility devices. In level 4 walking ability is severely limited even with assistive devices and finally in level 5 the voluntary control of movement and the ability to maintain head and neck position against gravity are completely impaired.
| Valuesa | |
|---|---|
| Age, mo | 83.96 ± 17.21 |
| Birth head circumference, cm | 30.46 ± 3.59 |
| Study time head circumference, cm | 48.69 ± 1.91 |
| Birthweight g | 1975.77 ± 867.26 |
| Time of CP diagnosis, mo | 8.50 ± 4.95 |
| Preterm labor, % | 65.4 (A)b |
| Age among preterm neonates, mo | 6.81 ± 0.55 |
| Caesarean section delivery, % | 69.2 |
| History of asphyxia, % | 57.7 |
| Abnormal Imaging findings, % | 53.8 (B)c |
aValues are expressed as mean ± SD.
b(A) Premature Rupture Of Membrane (PROM) was the most common cause of premature delivery comprising 60% of preterm cases while gestational diabetes mellitus and gestational hypertension comprises 26.6% and 13.3% of causes of premature delivery respectively.
c(B) From 14 patients with abnormal imaging findings 12 of them had periventricular leukomalacia in their imaging (85.8%) and 1 of them had ventriculomegaly (7.1%) while 1 patient had both abnormalities in the imaging (7.1%).
| Types of CP | Number of Patients |
|---|---|
| Physiologic | 23 Spastic; 3 Mixed |
| Anatomic | 3 Diplegic; 6 Hemiplegic; a 17 Quadriplegic |
| Functional | 1 in 2nd level; 4 in 3rd level; 8 in 4th level; 13 in 5th level |
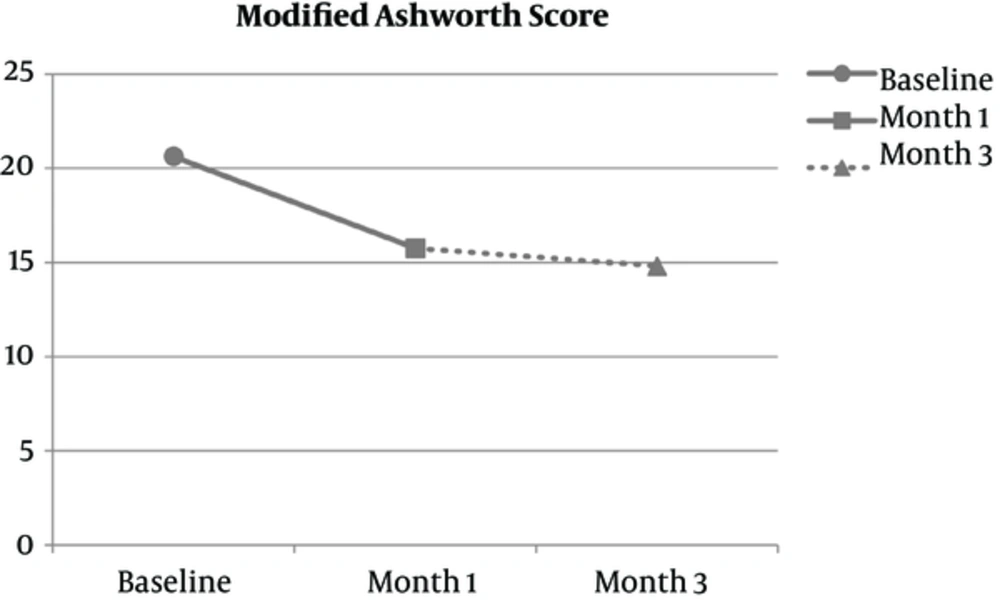
As mentioned before, MAS was assessed at baseline, 1 month and 3 months after therapy. The results are shown in Figure 2. Total decline in MAS was 5.42 ± 5.33 in 1 month and 5.81 ± 2.74 in 3 months of Cerebrolysin injections. These figures are dubbed as 25.05 ± 3.49 percent decrease at first month which reaches 26.08 ± 2.33 percent at third month in comparison to baseline. The difference between MAS at baseline and 1 month after therapy was significant (P = 0.000) but there is no significant difference between first month and third month of injections (P = 0.755). Also analysis indicates that absolute reduction of MAS is associated with the age at the beginning of treatment (P = 0.037).
5. Discussion
This is one of the few studies, investigating the efficacy of Cerebrolysin on motor improvement in CP patients. In our trial, MAS was the most important parameter for the evaluation of spasticity. In contrast to many other studies on Cerebrolysin, our trial was designed in a form to not only assess the efficacy of this drug in patients with CP, but also evaluate the appropriate protocol of drug use for achieving better results. For reaching the latter goal, we altered the method of administration during the period of therapy. To sum up, in our study MAS was assessed at baseline, one month and three months after therapy. Results showed considerable improvement after the first month – with 5 injections per week – but no significant recovery was observed in month 3 – with 1 injection per week – in comparison to month 1. It seems that with less Cerebrolysin shots in second and third months of treatment the process of progress was diminished. In other words, therapy continuation with less frequent injections maintained the primary level of amelioration, but did not make it better. Also these results can be interpreted as a plateau level of therapeutic effects after the first month of treatment with Cerebrolysin. The authenticity of these assumptions requires further studies with longer periods of assessment.
There are few articles writing about the effects of Cerebrolysin on different aspects of patients with CP. A study in 2017 reported that combination therapy of cerebrolysin and rehabilitation improved gross motor function of CP patients considerably (20). Liang X used point injection of Cerebrolysin to treat speech and cognitive function of children with CP (21). He realized that the earlier patient receives treatment, the better clinical outcome would be. This point was achieved in our study too. Another study showed the efficacy of Cerebrolysin for prelinguistic communication defects in infants with primary brain insult (22). This trial reported two new cases with intractable seizures when the treatment began. This condition was controlled with discontinuation of therapy. Until now considering seizure as a complication of Cerebrolysin is in controversy. Some studies’ results (23, 24) indicated that not only patients who use Cerebrolysin are not susceptible to seizure, but also it may have some protective effect for this condition. In our trial, no seizure was reported during the therapy. Also the dosage of 0.1 cc/kg of the drug was well tolerated in all of our cases. Other anticipated complications such as dizziness, agitation or skin flush were not observed either.
But how Cerebrolysin may improve the function of patients with CP and neurodegenerative disorders? Many animal studies have been performed until now; but the mechanism of action is still not totally clear. It seems that Cerebrolysin has a neuroimmunotrophic activity reducing the extent of inflammation and increasing the death of neurons, which are under pathological conditions such as those observed in neurodegenerative disorders (24). Different studies on cultured cortical neurons of chicken embryo show that besides having anti-apoptotic and protective effects; Cerebrolysin may stimulate the overgrowth of neuritis (25, 26). More studies in rats showed the increase of nerve growth factor’s expression and its receptor after intracerebroventricular infusion (27). Overall, these studies and other similar trials can, somewhat but not completely, explain why Cerebrolysin is an effective medication for neurodegenerative disorders including CP.
5.1. Conclusions
Cerebrolysin may at least for a short-term – up to 3 months – improve the spasticity of CP children. Moreover, the earlier Cerebrolysin therapy begins the more it may be beneficial.
5.2. Suggestions
Longer follow-up time, double blind placebo control and considering other developmental aspects especially cognitive and speech are our suggestions for further studies. Moreover, the effects of Cerebrolysin on function and participation of CP patients should be investigated in future studies.