1. Background
Thyroid hemiagenesis (THA) is a rare congenital developmental abnormality, where one of the thyroid lobes fails to develop (1). The etiology of THA and other thyroid dysgenesis (TD) is largely unknown (2, 3). Thus, factors that disturb the process by influencing the lobulation of the thyroid anlage are yet to be unraveled (4, 5). Although the occurrence of THA and other TD in families suggests a genetic background (6), THA usually occurs as sporadic condition (7). The process of thyroid bilobation lasts until 10 - 12th week of gestation (8). Thus, the exposure of the pregnant women to some environmental factor in the first trimester might disturb that process.
The process of thyroid bilobation proceeds in parallel with the development of the blood vessels from the third pharyngeal arch (9). A recent report demonstrated by CT-Angiography agenesis of the thyroid artery on the agenetic side in patients with THA. Therefore, the lack of one thyroid lobe may result from inadequate blood supply at early stages of embryogenesis (10). We cannot rule out a role of other unknown factors, which may modulate expression of the final phenotype. Overall, it seems probable that TD results from a complex interplay between genetic and environmental factors (1).
An interesting and promising concept was that environmental factors during pregnancy might interrupt the thyroid embryogenesis, and therefore result in a misdevelopment of the fetal thyroid gland. However, data from previous studies on association between the season of birth and the incidence of TD (mainly agenesis and ectopy) causing congenital hypothyroidism (CH) are conflicting (11-19). So far, seasonality of birth have been linked to several autoimmune endocrine diseases (13, 20-22). To date, seasonality of births have not been analyzed in regard to the presence of THA yet. Thus, the aim of this study was to assess whether there is any preference for seasonality in the dates of birth of patients with THA in comparison to general population.
2. Methods
2.1. Patients
The studied group consisted of 105 consecutive patients diagnosed with THA at two academic tertiary reference centers in the years 2008 - 2016. There were 14 men and 91 women, aged 2 - 80 years. The median age at diagnosis was 28 years old for women and 34 for men. The diagnosis of THA was initially made by thyroid ultrasound examination and then confirmed by thyroid I131 scintiscan. The diagnosis of THA was established when one of the thyroid lobes or lobe with isthmus could not be visualized on ultrasound examination. Patients following thyroidectomy, radioiodine therapy or autoimmune destructive thyroiditis were excluded from analysis. Absence of the lobe was confirmed by demonstration of a unilateral isotope uptake on thyroid I131 scintiscan. One thyroid lobe hypoplasia or other types of TD constituted the exclusion criteria. The control data regarding total live births in Poland were derived from the Polish Statistical Annals for the years 1980, 1990, 2000, 2010, and 2012 (n = 2 421 384).
2.2. Methods
All procedures performed in our study were in accordance with the ethical standards of our institutional Bioethical Committee of Poznan University of Medical Sciences and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. An informed consent was obtained from all individual participants included in the study after full explanation of the purpose and nature of all procedures used.
The dates of birth of patients diagnosed with THA were analyzed and compared to the dates of birth for the Polish population. Both groups were subdivided according to the month of birth, season of birth (spring, summer, autumn, winter) and quarter (1 - 4) of the year. The seasons of the year in the study were established as follows: spring (March, April, May), summer (June, July, August), autumn (September, October, November) and winter (December, January, February). The quarters of the year were defined accordingly: 1st (January, February, March,), 2nd (April, May, June), 3rd (July, August, September) and 4th (October, November, December). The differences between the groups were analyzed statistically. Statistical analysis was conducted using MedCalc version 16.8.4. (MedCalc software bvba) and TSA Cosinor software (Expert Soft Technologie). Month-by-month variation was screened for using chi-square test for independece, with odds ratios (ORs) and 95% confidence intervals (CIs) calculated, to compare the birth rates for each month in subjects with THA and the control population. A Cosinor model was used to estimate the annual seasonal trend in the month-of-birth distribution across all 12 months. A P-value of less than 0.05 was regarded as significant.
3. Results
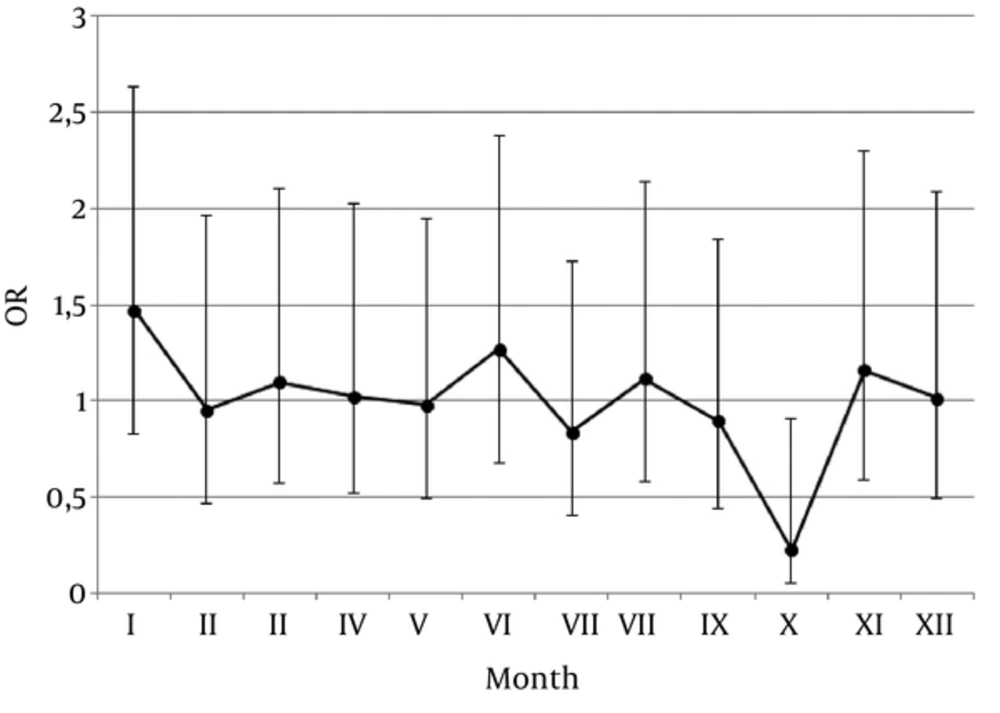
A percentage of patients with THA and control subjects born in particular months of the year as well as observed differences are presented in Table 1. Odds ratios (OR) and 95% confidence interval (CI) values for the chance to be born in particular month for the patients with THA were presented in Figure 1. An analysis of the months of birth revealed that patients with THA were born significantly less often in October than control subjects (P = 0.0217). Furthermore, a studied THA population was tested using Cosinor model, however the observed variations in the dates of birth were considered not significant (P = 0.3714).
| Months of Birth | Control Subjects (%) | THA Patients (%) | ORa | 95%CIa | P Value | |
|---|---|---|---|---|---|---|
| I | 8.76 | 12.38 | 1.47 | 0.82 | 2.63 | 0.1891 |
| II | 7.96 | 7.62 | 0.95 | 0.46 | 1.96 | 0.8975 |
| II | 8.76 | 9.52 | 1.10 | 0.57 | 2.10 | 0.7818 |
| IV | 8.39 | 8.57 | 1.02 | 0.52 | 2.03 | 0.9480 |
| V | 8.73 | 8.57 | 0.98 | 0.49 | 1.94 | 0.9540 |
| VI | 8.43 | 10.48 | 1.27 | 0.68 | 2.37 | 0.4504 |
| VII | 8.95 | 7.62 | 0.84 | 0.41 | 1.73 | 0.6325 |
| VII | 8.62 | 9.52 | 1.12 | 0.58 | 2.14 | 0.7405 |
| IX | 8.43 | 7.62 | 0.90 | 0.44 | 1.84 | 0.7658 |
| X | 7.97 | 1.90 | 0.22 | 0.06 | 0.91 | 0.0217 |
| XI | 7.49 | 8.57 | 1.16 | 0.58 | 2.29 | 0.6730 |
| XII | 7.51 | 7.62 | 1.02 | 0.49 | 2.09 | 0.9675 |
Abbreviations: OR, odds ratios; CI, confidence interval.
aValues for the Chance to be Born in Particular Month for the Patients with THA and Control Subjects.
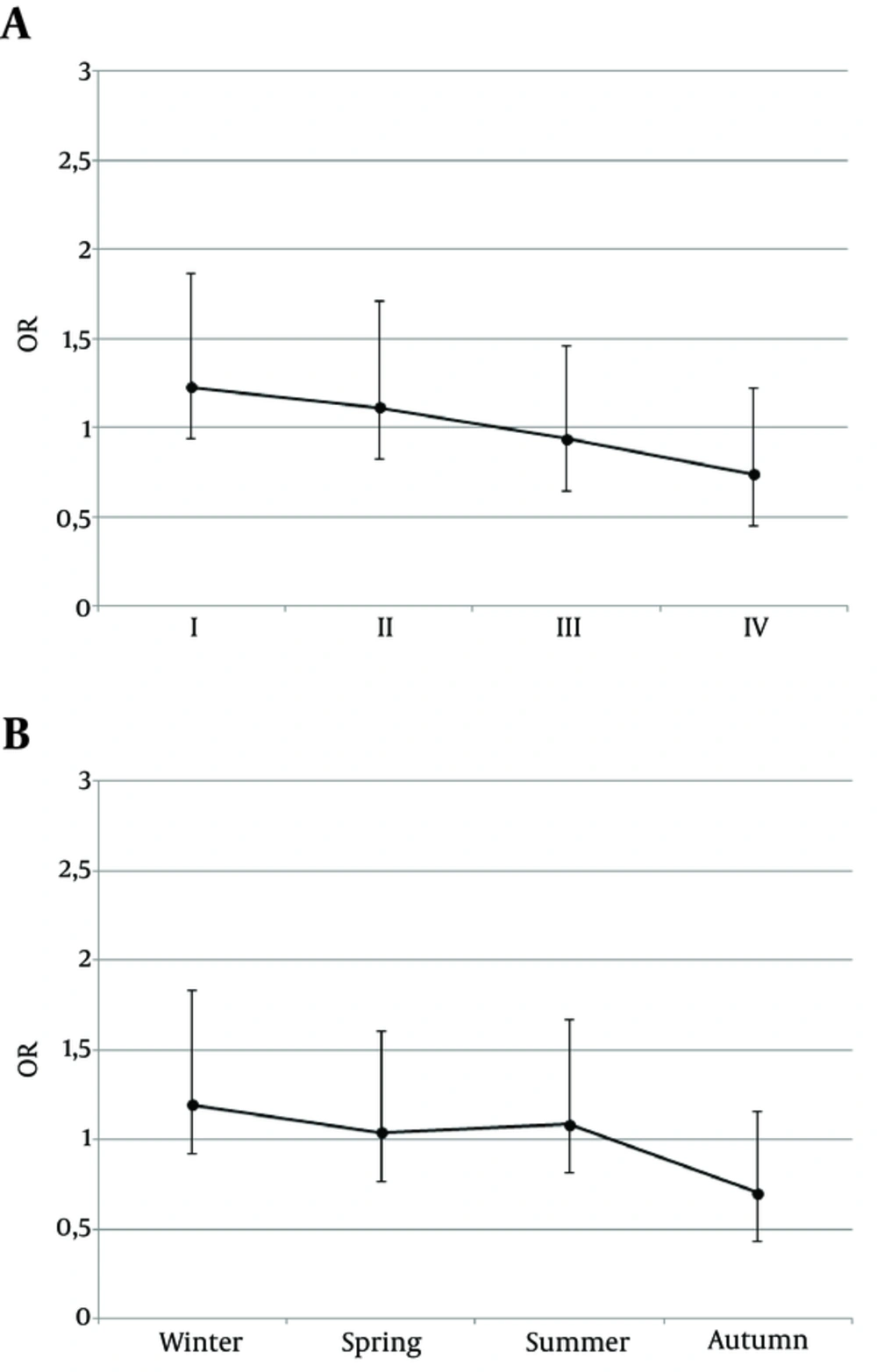
Moreover, a separate analysis was performed when patients were divided according to the quarters of the year. There were: 31 patients with THA born in the 1st quarter, 29 born in the 2nd quarter, 26 born in the 3rd quarter and 19 born in the 4th quarter. If the seasons of the year are concerned: 29, 28, 29 and 19 patients with THA were born in winter, spring, summer and autumn, respectively. Although a trend is observed, that more patients with THA were born in the 1st quarter of the year (Figure 2A) and in winter (Figure 2B), while THA patients were less often born in autumn or in 4th quarter of the year, the difference did not reach statistical significance.
4. Discussion
Several studies on the association between the month or season of birth and the risk for TD have been conducted so far. However, it mainly included patients diagnosed with CH, presenting either thyroid ectopy, agenesis or severe thyroid hypoplasia. To date, such an analysis has never been performed for patients with THA. Similarly to our cohort, the anomaly is usually detected incidentally in adulthood (1). Hence, collection of a large cohort of patients with THA is challenging. Severe types of TD result from disturbances of thyroid primordial development or downward migration. However, whether the same factors influence thyroid bilobation remains unknown.
So far conducted studies on the association of birth season and the risk of TD provide conflicting results, likewise the data on the period of a year predisposing to the development of TD. One of the first large cohort studies reporting a seasonal variation in the incidence of CH was performed in Finland by Virtanen et al. (19). The study on Japanese population by Nakamizo et al. in a - similar to ours - group of 108 patients, revealed the highest incidence of CH in February, and the lowest in May (16). In our cohort, the highest incidence of THA was noted in January, and the lowest in October. The number of CH patients born in winter (December-February) was the largest among the four seasons, if compared to spring or autumn (16). These results are in accordance with our observations for THA. Miyai et al. analyzed newborns with CH caused by TD in a period of 14 years, and the incidence of CH appeared significantly higher in late autumn (from October to December) (15). These results stay in contrast to our study, as we observed the lowest incidence of THA in this period of the year. What is interesting, in the study by Miyai et al. the prevalence of dysgenetic CH was found to be higher about 9 - 10 months following the emergence of influenza. Authors speculated that this might be related to the maternal viral infection.
Another study performed on the British population of 1128632 live newborns over a period of 16 years demonstrated a significantly increased (1:2323 live births) incidence of CH between October and December, if compared to average 1:2924 (12). In contrast, higher incidence of CH was observed in the Japanese population between May and July in the years 1957 - 1976, and for October–December for the next five years (14). These results are in contrast to our findings, where October was the month of the lowest incidence of THA.
On the other hand, several authors were not able to find any relationship between the date of birth and the incidence of TD. Rosenthal et al. did not demonstrate seasonal variability in the incidence of CH in the study on North-West England population (18). The conclusions were consistent with the Dutch research (17). In the recent paper by Deladoey et al. a cohort of 424 subjects diagnosed with CH due to TD in a period of 16 years was analyzed and the distribution of the months of birth was accidental, without any preponderance to particular season of the year (11).
The impact of the month-of-birth on the risk of several diseases has been studied also for autoimmune disorders, including Addison disease, or type 1 diabetes, with the risky period of a year in autumn/winter months (13, 20-22). Possible explanations for such an asymmetric distribution of the dates of birth include the influence of seasonal maternal viral infections or vitamin D deficiency on the developing progeny’s innate immune system (23). It is also suspected that hitherto undefined environmental factor of seasonal appearance may affect the process of organogenesis, including the development of the thyroid gland.
On the other hand, cases of familial occurrence of different TD including THA and reported discordance of monozygotic twins if the thyroid morphology is concerned, may argue against the dominant role of environmental factors in the pathogenesis of thyroid developmental disturbances (24, 25). However, still the modifying impact cannot be ruled out.
In interpretation of our findings we need to note potential limitations of our study. The incidence of THA varies according to different studies and is estimated at 0.05 - 0.5% of the general population (1). However, due to its relatively oligosymptomatic course, it is usually detected incidentally. Although our group of patients with THA is the largest reported to date, from the statistical point of view our sample size is still relatively small, what might potentially limit the power of the study. Secondly, the subjects in our study were not recruited from a formal national or regional registry, and represent consecutive patients diagnosed at two tertiary reference centres. This could potentially be the reason for a bias. Moreover, in majority of patients we were not able to verify whether they were born at term. Lastly, our controls obtained from the Polish Statistical Annals inevitably include subjects with THA. However, given the rarity of the disease, the expected number of THA cases in the control population is probably too small to affect the results. The gender distribution of our cohort of patients with THA is also different from that of general population. However, it does not seem to importantly influence the results.
4.1. Conclusions
To the best of authors’ knowledge, this is the first analysis of birth seasonality performed on a group of patients with THA. Our study demonstrates that month of birth may exert some effect on the risk of developing THA. Although a trend is observed that more patients with THA were born in the 1st quarter of the year and in winter, while THA patients were less often born in autumn or in 4th quarter of the year, the difference did not reach statistical significance. Studies on larger cohort of subjects are needed to confirm the observation. The results suggest a potential modifying effect of environmental factors in the pathogenesis of THA. The exact factor remains unknown and further studies are warranted to provide an explanation for the observed phenomenon. Identification of environmental factors potentially disturbing the normal thyroid development would allow proposing methods of prevention.